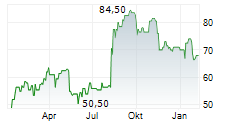
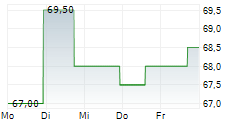
WASHINGTON (dpa-AFX) - ANI Pharmaceuticals, Inc. (ANIP) announced Friday that the U.S. Food and Drug Administration (FDA) has approved an expanded label for ILUVIEN (fluocinolone acetonide intravitreal implant) that includes an indication for the treatment of chronic non-infectious uveitis affecting the posterior segment of the eye (NIU-PS).
The approval also includes other updates to the label including to the Warnings and Precautions section.
The Company plans to market ILUVIEN for chronic NIU-PS in addition to its current indication of diabetic macular edema (DME) in the U.S. ILUVIEN is already approved for both DME and NIU-PS outside the U.S., including in seventeen European countries.
ANI previously announced that it extended its supply agreement for ILUVIEN with a subsidiary of Siegfried Holding AG through 2029. Siegfried and ANI also agreed to upgrade equipment on the existing manufacturing line and significantly expand capacity.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News