More consistent accumulation demonstrated in bloodstream over a one-week duration with once-daily DehydraTECH-tirzepatide oral capsules as compared to once-weekly injection of Zepbound®
As previously announced, oral DehydraTECH-tirzepatide also reduced adverse events by 47% compared to injected Zepbound®
Lexaria's oral capsules worthy of expanded investigation as a viable alternative to injected tirzepatide.
KELOWNA, BC / ACCESS Newswire / March 18, 2025 / Lexaria Bioscience Corp. (NASDAQ:LEXX)(NASDAQ:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms, is pleased to announce positive pharmacokinetic ("PK") results from Human Study #3 or GLP-1-H24-3 (the "Study"), comparing an oral version of DehydraTECH-processed Zepbound® ("DehydraTECH-tirzepatide") to conventional injected Zepbound®.
Zepbound® is currently only available as a once-weekly injection for weight loss. It is not sold by Eli Lilly in any oral format. Lexaria's Study was designed to discover whether the active drug within Zepbound® - tirzepatide - could be administered using Lexaria's patented DehydraTECH drug delivery technology via simple oral capsules in order to deliver a useful quantity of tirzepatide into the human bloodstream, as well as provide a viable alternative to disliked injections. The results were unexpectedly positive, showing that orally delivered DehydraTECH-tirzepatide reached roughly equal end of Study blood-concentration levels as the injected drug.
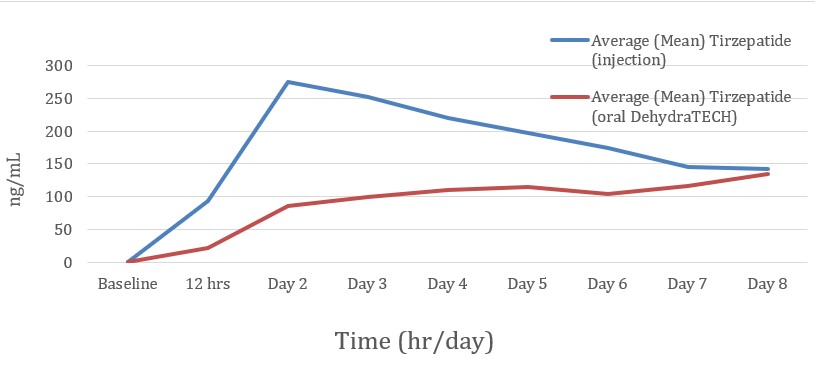
During the 8-day Study, data was successfully collected from 10 people who were dosed with a single weekly injection of Zepbound®, and from 9 people who were dosed daily over the same one-week duration with DehydraTECH-tirzepatide capsules.
In general, the peak levels of blood delivery of the injected Zepbound® were, for the most part, higher than that of the DehydraTECH-tirzepatide, but not in all cases. The injected Zepbound® typically reached a peak level within blood on the 2nd day of the Study and subsequently declined. Conversely, the DehydraTECH-tirzepatide blood levels increased steadily and more consistently each day of the Study, avoided any abrupt peaks or declines, and were generally still rising on Day 8, the final day of the Study. Of those receiving the Zepbound® injection, 8 of 10 people (80%) experienced peak levels on Day 2 of the Study and subsequently experienced declining levels, whereas of those receiving the DehydraTECH-tirzepatide, 4 of 8 people (50%) experienced their peak levels on the final day of the Study, indicating that their levels were still rising at the conclusion of the Study.
As announced on January 14, 2025, during the Study DehydraTECH-tirzepatide also evidenced reduced side effects, while achieving comparable concluding glycemic performance indicators. The injected Zepbound® produced a total of 38 adverse events during the Study, whereas the oral DehydraTECH-tirzepatide produced only 20, or 47% fewer, adverse events along with a 54% reduction in gastrointestinal side effects as compared to the Zepbound®. Furthermore, the DehydraTECH-tirzepatide capsules produced a comparable reduction in blood glucose together with a similar increase in blood insulin from baseline to Day 8 of the Study.
"Lexaria's first-ever study of oral DehydraTECH-tirzepatide has far exceeded our expectations," said Richard Christopher, CEO of Lexaria. "We have succeeded in demonstrating all 3 of our main objectives; reduced side effects with similar efficacy and similar blood-delivery levels as compared to injected tirzepatide by the end of the Study. Our ongoing 12-week study in Australia is well positioned to further evaluate the effectiveness of DehydraTECH over an extended dosing duration and potentially establish Lexaria as a global player in oral delivery within the fast-growing GLP-1 weight loss and diabetes control markets."
All the results of this study - reduced side effects, comparable end of Study blood sugar control and measured drug in bloodstream - are extremely encouraging and support our decision to further evaluate DehydraTECH-tirzepatide in our ongoing Australian Phase 1b registrational study (GLP-1-H24-4). In that 12-week study, DehydraTECH-tirzepatide will be dosed at the same 20mg/day level utilized in Human Study #3 for the initial 4 weeks of treatment, escalating further to 40mg/day over the last 8 weeks of treatment, thereby allowing Lexaria to potentially demonstrate further increased delivery and efficacy relative to the present Study.
Lexaria looks forward to the opportunity to assess the very important steady-state blood levels which DehydraTECH-tirzepatide administered via its oral capsules will achieve over an extended dosing duration in study GLP-1-H24-4 as they relate to published figures for sustained injectable tirzepatide dosing, given the fact that blood levels from DehydraTECH-tirzepatide witnessed in the present Study were continuing to ascend at Day 8.
There is no oral version of tirzepatide sold in the world today, as it is administered only by injection (Zepbound® and Mounjaro®). Lexaria has previously completed other research with oral DehydraTECH-processed semaglutide, sold by Novo Nordisk®, which is the only GLP-1 drug that is currently available as both an oral (Rybelsus®) and an injectable (Ozempic® and Wegovy®). This research yielded similar findings wherein Lexaria's DehydraTECH-processed semaglutide evidenced certain improvements in oral delivery compared to Rybelsus®. Lexaria believes that an effective oral version of tirzepatide with fewer adverse events than the current injectable versions, could be highly valued.
As noted, Rybelsus® is the only orally delivered GLP-1 drug on the market today. Rybelsus® uses a proprietary drug delivery technology called salcaprozate sodium ("SNAC"), that Novo Nordisk paid US$1.8 billion to acquire, to enable efficacy in an oral delivery format. Lexaria notes that, of course, there was no SNAC present in either the Zepbound® or in Lexaria's DehydraTECH-tirzepatide in the present Study, showcasing that Lexaria's wholly owned DehydraTECH technology has now demonstrated its ability to successfully deliver 3 of the world's best-selling weight-loss and diabetes control drugs: tirzepatide, liraglutide, and semaglutide across its animal and human GLP-1 studies conducted to-date.
About the Study
Many design characteristics of the Study, also referred to as Study GLP-1-H24-3, are similar to Lexaria's initial GLP-1 human pilot study #1, investigating the dual agonist GLP-1/GIP drug tirzepatide in this Study instead of the GLP-1 agonist semaglutide from human pilot study #1. The DehydraTECH-tirzepatide test articles were compound formulated using Zepbound®, strictly for research purposes, and dosed orally to the subjects under fasted conditions. The Study was designed to measure tolerability and side effects, blood levels of tirzepatide, and blood glucose and insulin levels. The DehydraTECH-tirzepatide composition was formulated at a strength of 20 mg tirzepatide administered orally daily for seven days followed through to the end of the eighth day post-dosing. The Zepbound® formulation had a strength of 2.5 mg tirzepatide administered once via injection with the subject monitored over the same eight-day total duration. Blood samples were taken multiple times during the first 12 hours post dosing on the first day of each treatment phase, with single blood samples taken daily thereafter through to a final blood draw taken 24 hours after the end of dosing (i.e., on the eighth day of the Study); and, all subjects were dosed under fasted conditions with a standardized meal fed to the test subjects at a point in time after dosing. Subjects were dosed with each test article following a randomized, cross over study design across two study phases, separated by a 4-6 week washout duration.
About Lexaria Bioscience Corp. & DehydraTECH
DehydraTECH is Lexaria's patented drug delivery formulation and processing platform technology which improves the way a wide variety of drugs enter the bloodstream, always through oral delivery. DehydraTECH has repeatedly evidenced the ability to increase bio-absorption, reduce side-effects, and deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 48 patents granted and additional patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
ir@lexariabioscience.com
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.



