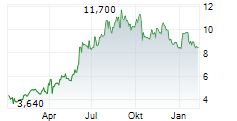
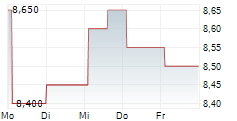
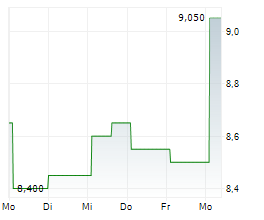
BEIJING (dpa-AFX) - Innovent Biologics Inc. and HUTCHMED (China) Limited announced that the FRUSICA-2 Phase 2/3 clinical trial, which evaluated sintilimab in combination with fruquintinib as second-line treatment for locally advanced or metastatic renal cell carcinoma (RCC) in China, has met its primary endpoint of progression free survival per RECIST 1.1 as assessed by blinded independent central review (BICR).
The companies noted that the combination of sintilimab and fruquintinib received conditional approval from China's National Medical Products Administration (NMPA) for the treatment of patients with advanced endometrial cancer with Mismatch Repair proficient (pMMR) tumors that have failed prior systemic therapy and are not candidates for curative surgery or radiation, based on data from the FRUSICA-1 study.
The FRUSICA-2 study is a randomized, open-label, active-controlled study to evaluate the efficacy and safety of sintilimab in combination with fruquintinib versus axitinib or everolimus monotherapy for the second-line treatment of advanced renal cell carcinoma.
In addition to the primary endpoint progression free survival, the combination also demonstrated improvements in secondary endpoints, including objective response rate (ORR) and duration of response (DoR).
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News