WASHINGTON (dpa-AFX) - Plus Therapeutics (PSTV) announced the FDA has conditionally accepted the company's new proprietary name, REYOBIQ, for its lead therapeutic candidate. The company noted that REYOBIQ or rhenium Re186 obisbemeda continues to be under clinical investigation for Leptomeningeal Metastases and Recurrent Glioblastoma.
'Branding is an important part of preparing for commercialization, and the establishment of the REYOBIQ brand will enable investigators, investors, and potential patients to connect with our rhenium-based radiotherapeutic beyond its chemical identity,' said Russ Havranek, Plus Therapeutics VP of Corporate Strategy and New Product Planning.
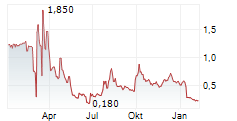
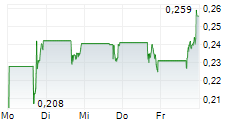
Shares of Plus Therapeutics are up 20% in pre-market trade on Thursday.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News