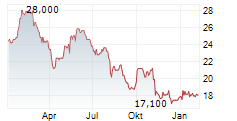
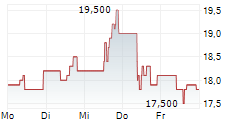
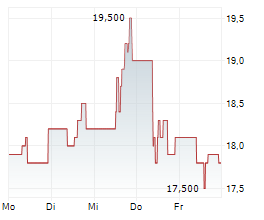
LONDON (dpa-AFX) - Hikma Pharmaceuticals (HIK) announced the approval of KLOXXADO Nasal Spray 8 mg, by Health Canada for the treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression, for adult patients. KLOXXADO Nasal Spray will be marketed and sold in Canada by Emergent BioSolutions under the terms of a recent six-year commercial agreement with Hikma, whereby Emergent will be responsible for all North America product sales and marketing.
Emergent will engage Canadian agencies, private payers and provincial formularies, and plans to make KLOXXADO available via prescription as early as 2026.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News