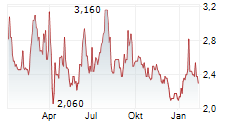
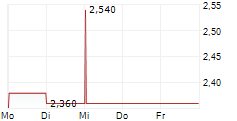
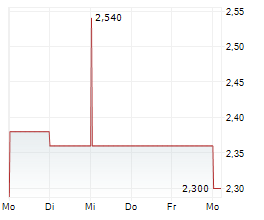
BEIJING (dpa-AFX) - HUTCHMED China (HCM) announced that the New Drug Application for TAZVERIK has been granted conditional approval in China for the treatment of adult patients with relapsed or refractory follicular lymphoma with EZH2 mutation who have received at least two prior systemic therapies. This marks the first nationwide regulatory approval for TAZVERIK in China.
HUTCHMED is responsible for the research, development, manufacturing and commercialization of TAZVERIK in China Mainland, Hong Kong, Macau and Taiwan. Epizyme will be the Marketing Authorization Holder of TAZVERIK in China.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News