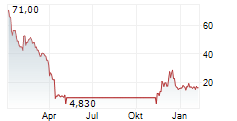
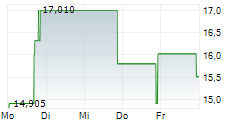
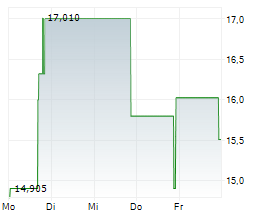
WASHINGTON (dpa-AFX) - Palatin Technologies (PTN) announced the FDA has granted orphan drug designation to PL7737, an oral treatment that activates the melanocortin-4 receptor, for leptin receptor deficiency, including obesity caused by this condition.
Carl Spana, President and CEO of Palatin, said: 'Currently, the only FDA-approved treatment for obesity due to leptin receptor deficiency is a daily injection. PL7737's oral form could provide a more convenient and effective option for these patients and others with rare genetic obesity disorders. We are also exploring PL7737 for hypothalamic obesity and plan to begin a Phase 1 SAD/MAD study in late 2025.'
Shares of Palatin Technologies are up 8% in pre-market trade on Tuesday.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News