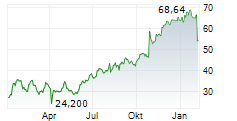
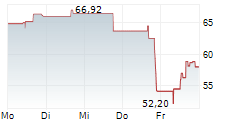
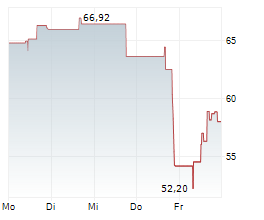
LONDON (dpa-AFX) - BridgeBio Pharma (BBIO) announced the Japanese Ministry of Health, Labour and Welfare has approved acoramidis, under the brand name Beyonttra, for the treatment of adults with transthyretin-mediated amyloid cardiomyopathy. The approval is based on positive results from a Phase 3 open-label, single-arm study conducted in Japan by Alexion, AstraZeneca Rare Disease, and the positive results from the global ATTRibute-CM Phase 3 study.
Alexion, AstraZeneca Rare Disease, maintains an exclusive license with BridgeBios affiliate, Eidos Therapeutics, Inc. to develop and commercialize acoramidis in Japan. BridgeBio will receive a $30 million milestone payment upon approval in Japan, as well as royalties in the low double digits on sales of acoramidis in Japan, with commercialization efforts planned in the first half of 2025.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News