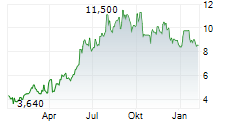
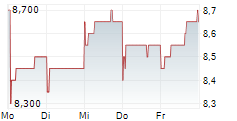
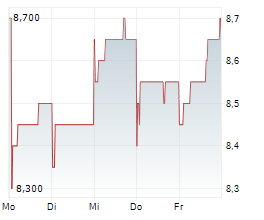
BEIJING (dpa-AFX) - Innovent Biologics Inc. (IVBXF.OB) announced that the Center for Drug Evaluation (CDE) of China's National Medical Products Administration (NMPA) has granted Breakthrough Therapy Designation (BTD) for IBI363, a first-in-class PD-1/IL-2?-bias bispecific antibody fusion protein, as monotherapy for the treatment of unresectable locally advanced or metastatic mucosal or acral melanoma who have not received prior systemic therapy.
Recently, the company initiated and dosed the first patient for IBI363 in its first pivotal study to evaluate the efficacy and safety of IBI363 monotherapy versus pembrolizumab (Keytruda) monotherapy in patients with unresectable, locally advanced or metastatic mucosal or acral melanoma who have not received prior systemic therapy.
In addition, IBI363 received two fast track designations (FTD) from the U.S. Food and Drug Administration (FDA), for the treatment of squamous non-small cell lung cancer and melanoma, respectively.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News