NEW YORK CITY (dpa-AFX) - Halozyme Therapeutics, Inc. (HALO), Monday announced that Bristol Myers Squibb (BMY) received a positive opinion from the Committee for Medicinal Products for Human Use of the European Medicines Agency, recommending approval of a new Opdivo subcutaneous formulation developed with the company's ENHANZE technology.
The new cancer drug delivery method was tested across multiple solid tumor indications in the European Union, demonstrating a faster and more flexible treatment option and helping alleviating pressure on healthcare system resources.
The regulatory agency's positive opinion is based on positive results from the Phase 3 CheckMate -67T trial.
Following this recent announcement, the CHMP opinion will now be reviewed by the European Commission, which is expected to approve the European extension of marketing authorization for the subcutaneous formulation of Opdivo by June 2, 2025.
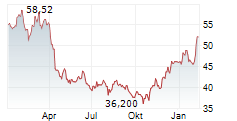
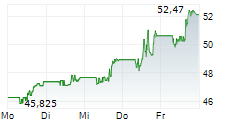
Currently, Halozyme's stock is dropping 1.02 percent, to $63.19 on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News