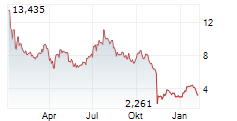
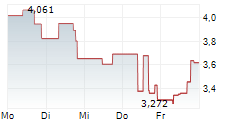
WASHINGTON (dpa-AFX) - Anavex Life Sciences Corp. (AVXL) announced that more than three years of continuous treatment with blarcamesine (ANAVEX 2-73) significantly improved clinical decline, demonstrating sustained and meaningful benefits for early Alzheimer's disease patients, according to results from the Phase IIb/III open-label extension trial.
The company noted that continued blarcamesine treatment up to four years demonstrated good comparative safety profile and no associated neuroimaging adverse events (no potentially fatal brain bleeding or brain swelling). There were no deaths related to the study drug.
The ATTENTION-AD (ANAVEX2-73-AD-EP-004) trial followed the 48-week ANAVEX2-73-AD-004 double-blind (DB) clinical trial, lasting up to 192 weeks, with an open-label extension (OLE) treatment duration of 96 weeks for participants in Canada and Europe and up to 144 weeks for participants in Australia to evaluate the safety and tolerability of blarcamesine and long-term effects of blarcamesine on cognition and function in participants with early Alzheimer's disease.
The delayed-start analysis for ADAS-Cog13 showed a significant difference between early start and late start treatment groups up to Week 192, favoring the early start group. Together these results suggest that participants who initiated treatment with blarcamesine earlier in their disease progression showed greater stability of cognitive function compared to those who did not initiate blarcamesine until about 1 year later.
In addition, to place these findings in context, an ADAS-Cog13 score difference between the treatment groups at Week 192 being larger than 2 points is considered a clinically meaningful improvement.
Similarly, the delayed-start analysis for ADCS-ADL showed numerically favorable results for the early start group over the late start group up to Week 192 and reached statistical significance.
An additional analysis, evaluating the therapeutic effect of continuous drug treatment versus treatment interruption between the end of double-blin trial and the beginning of the open-label extension trial versus a longer drug interruption, confirmed the beneficial blarcamesine treatment effect.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News