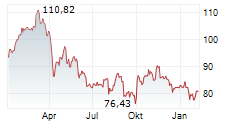
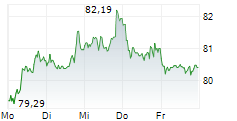
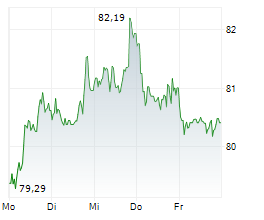
PARIS (dpa-AFX) - French drugmajor Sanofi (SNY) announced Tuesday new progress from its mid- to late-stage respiratory pipeline, including preliminary phase 2 results for amlitelimab in adults with moderate-to-severe asthma.
In its statement, Sanofi announced that preliminary results from the TIDE-Asthma phase 2 study show that the primary endpoint of annualized exacerbation rate at week 48 was not met at the highest dose level, leading to nominal significance at the medium and low doses.
However, the study demonstrated amlitelimab's compelling efficacy in heterogeneous inflammatory asthma, potentially representing a breakthrough for this underserved patient population if observed in later studies.
Further, Lunsekimig is now targeting chronic rhinosinusitis and chronic obstructive pulmonary disease or COPD in addition to asthma; and Itepekimab is expanding into chronic rhinosinusitis along with COPD and bronchiectasis.
The company plans phase 3 readouts in COPD in the second half 2025 and bronchiectasis in 2026.
Regarding phase 2 study, the firm noted that treatment with amlitelimab led to nominally significant and clinically meaningful reductions in asthma exacerbations at the medium dose tested and a numerically greater reduction in exacerbations at the high dose at week 60.
The study also demonstrated nominally significant and clinically meaningful improvement in secondary endpoints of lung function and asthma control. Notably, in a patient sub-group defined by biomarkers, amlitelimab showed nominally significant and clinically meaningful improvements in exacerbations, lung function and asthma control at week 60.
According to Sanofi, these results demonstrate that amlitelimab has potential to improve key disease outcomes in asthma patients with continued unmet need. The phase 3 program is currently being planned.
Houman Ashrafian, Executive Vice President, Head of Research & Development, said, 'We are pleased by the significant progress we have made with our pipeline across respiratory indications. In asthma, amlitelimab shows potential as an effective, long-acting medicine, including in patients with moderate-to-severe heterogenous inflammation. If the preliminary effect we have seen is confirmed in phase 3 studies, amlitelimab could become a differentiated treatment option in asthma. These data validate our strategy to advance innovative science and provide new solutions for patients with challenging-to-treat respiratory diseases.'
The company plans to reveal full and final results at a forthcoming medical meeting.
Further, Sanofi noted that Lunsekimig has the potential for broader use in COPD, and is being explored in a broad population of asthma patients, regardless of their inflammation and severity status.
The readout of the AIRCULES phase 2 study in moderate to severe asthma is anticipated in 2026 while the AIRLYMPUS phase 2 study in high-risk asthma was initiated in the fourth quarter last year.
Further, the readout of the phase 2 study in patients with chronic rhinosinusitis with nasal polyps is anticipated in 2026, and a phase 2/3 study in COPD is anticipated to begin in 2025.
Regarding Itepekimab, the company said it is expanding clinical studies beyond COPD into chronic rhinosinusitis.
Sanofi added that, in partnership with Regeneron, two phase 3 studies in patients with chronic rhinosinusitis with nasal polyps (CRSwNP), CEREN 1 and CEREN 2 and one phase 2 study in patients with chronic rhinosinusitis without nasal polyps (CRSsNP) ) were initiated in Q1 2025.
Itepekimab is currently being explored in patients with COPD in two phase 3 studies AERIFY-1 and AERIFY-2 with the readout anticipated in H2 2025, and in one phase 2 study, AERIFY-3, with the readout anticipated in H2 2025.
Finally, itepekimab is being explored in a phase 2 study in bronchiectasis, with the readout anticipated in 2026.
For More Such Health News, visit rttnews.com
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News