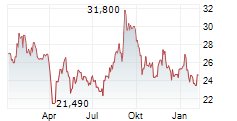
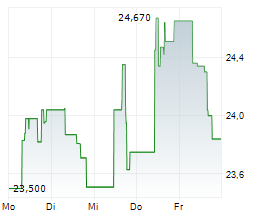
WESTON (dpa-AFX) - Eisai Co., Ltd. (ESALF.PK) and Biogen Inc. (BIIB) announced that the European Commission has granted the amyloid-beta (A) monoclonal antibody Leqembi (lecanemab) Marketing Authorization in the European Union.
Lecanemab is indicated for the treatment of adult patients with a clinical diagnosis of mild cognitive impairment (MCI) and mild dementia due to AD (early AD) who are apolipoprotein E 4 (ApoE 4*) non-carriers or heterozygotes with confirmed amyloid pathology.1 The lecanemab MA applies to all 27 EU Member States as well as Iceland, Liechtenstein, and Norway.
Lecanemab is the only approved A monoclonal antibody that preferentially binds and clears toxic protofibrils** (soluble A aggregates), in addition to targeting and reducing A plaques (insoluble A aggregates). Protofibrils are a key toxic form of A that accumulate in the brain and cause neuronal injury.
The European Commission's decision makes lecanemab the first treatment option in the EU that can slow the progression of early Alzheimer's disease.
For More Such Health News, visit rttnews.com.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News