WASHINGTON (dpa-AFX) - Regeneron Pharmaceuticals, Inc. (REGN), Thursday announced that the U.S. Food and Drug Administration has accepted for Priority Review the supplemental Biologics License Application for EYLEA HD (aflibercept) Injection 8 mg for the treatment of retinal vein occlusion.
The FDA target action date is August 19, 2025, following the use of a Priority Review voucher, the company added.
The application seeks approval for two areas - treatment of macular edema following retinal vein occlusion, and for broadening the dosing schedule to include every 4-week (monthly) dosing across approved indications.
If approved, EYLEA HD would be the first and only treatment for RVO indicated for up to every 8-week dosing after an initial monthly dosing period, reducing the number of injections by half compared to all other anti-VEGF therapies.
The application is backed by data from across the EYLEA HD clinical program, including the Phase 3 QUASAR trial, which met its primary endpoint at 36 weeks.
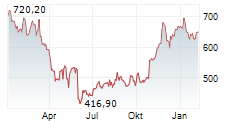
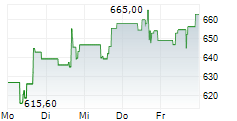
In the pre-market hours, Regeneron's stock is trading at $547.72, down 0.28 percent on the Nasdaq.
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News