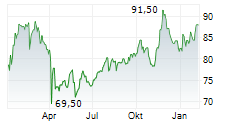
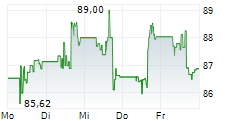
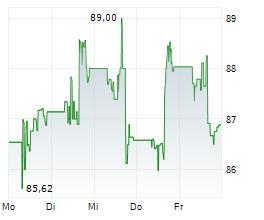
WASHINGTON (dpa-AFX) - Medtronic plc (MDT), Thursday announced it has submitted 510(k) applications to the U.S. Food and Drug Administration (FDA) seeking clearance for an interoperable pump.
FDA clearance of this pump would pave the way for system integration with a continuous glucose monitoring (CGM) sensor based on Abbott's most advanced CGM platform.
The submissions included a 510(k) application for its MiniMedT 780G pump as an alternate controller enabled (ACE) insulin pump and a separate 510(k) application for its SmartGuardT algorithm as an interoperable automated glycemic controller (iAGC).
'We understand how meaningful these advancements are, and we're working with urgency to bring enhanced CGM options to our customers,' said Que Dallara, EVP & president, Medtronic Diabetes. 'This collaboration with Abbott marks an important step forward in providing innovative solutions and more choice for our customers.'
Copyright(c) 2025 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2025 AFX News