| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
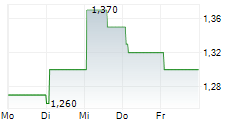
| 1,360 | 1,380 | 13:04 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 13.01. | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - FDA ACCEPTANCE OF THE NDA FOR PIMICOTINIB FOR THE TREATMENT OF TGCT | 1 | HKEx | ||
| 13.01. | Abbisko Therapeutics Announces FDA Acceptance Of Pimicotinib NDA For Tenosynovial Giant Cell Tumor | 419 | AFX News | HONG KONG (dpa-AFX) - Abbisko Therapeutics Co., Ltd. announced that the U.S. Food and Drug Administration (FDA) has formally accepted the New Drug Application (NDA) for pimicotinib (ABSK021).... ► Artikel lesen | |
| 13.01. | Abbisko Therapeutics Announces FDA Acceptance of the NDA for Pimicotinib for the Treatment of Tenosynovial Giant Cell Tumor | 239 | PR Newswire | SHANGHAI, Jan. 12, 2026 /PRNewswire/ -- 13 January 2026 (Beijing Time), Abbisko Therapeutics Co., Ltd. ("Abbisko Therapeutics" hereafter, HKEX code: 02256.HK) announced... ► Artikel lesen | |
| 24.12.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - IND CLEARANCE FROM THE NMPA FOR ORAL SMALL MOLECULE KRAS G12D INHIBITOR ABSK141 | 5 | HKEx | ||
| 22.12.25 | Abbisko Therapeutics Co., Ltd.: Abbisko Therapeutics' CSF-1R Inhibitor Pimicotinib Approved by the China NMPA, Addressing the Treatment Gap for Tenosynovial Giant Cell Tumor | 247 | GlobeNewswire (Europe) | Based on the positive global Phase III MANEUVER study, the National Medical Products Administration (NMPA) has granted the world's first regulatory approval for pimicotinib, the first domestically... ► Artikel lesen | |
| 22.12.25 | ABBISKO-B (02256): INSIDE INFORMATION - ABBISKO THERAPEUTICS' CSF-1R INHIBITOR PIMICOTINIB APPROVED BY THE CHINA NMPA | 3 | HKEx | ||
| 16.12.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - ABBISKO THERAPEUTICS COMPLETES FIRST PATIENT DOSING OF ABSK061, FGFR2/3 INHIBITOR, FOR THE TREATMENT OF ACHONDROPLASIA | 1 | HKEx | ||
| ABBISKO CAYMAN Aktie jetzt für 0€ handeln | |||||
| 08.12.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - ABBISKO THERAPEUTICS PRESENTS PRELIMINARY PHASE II RESULTS OF ORAL PD-L1 INHIBITOR ABSK043 COMBINED WITH EGFR ... | 1 | HKEx | ||
| 01.12.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - IND CLEARANCE FROM THE FDA FOR ORAL SMALL MOLECULE KRAS G12D INHIBITOR ABSK141 | 1 | HKEx | ||
| 17.11.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - ABBISKO THERAPEUTICS PRESENTS LONGER-TERM EFFICACY AND SAFETY OUTCOMES FROM PHASE III MANEUVER STUDY OF PIMICOTINIB ... | 1 | HKEx | ||
| 03.11.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - ABBISKO THERAPEUTICS COMPLETES FIRST PATIENT DOSING IN A PHASE II STUDY OF ABSK043, AN ORAL SMALL-MOLECULE ... | 1 | HKEx | ||
| 27.10.25 | Goldman Sachs initiates Abbisko Therapeutics stock with Buy rating | 3 | Investing.com | ||
| 27.10.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - ABBISKO THERAPEUTICS PRESENTED PRECLINICAL FINDINGS ON NOVEL CDK4/2 INHIBITOR AND SMARCA2 PROTAC DEGRADER ... | 1 | HKEx | ||
| 20.10.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - ABBISKO THERAPEUTICS PRESENTS LONGER-TERM EFFICACY AND SAFETY OUTCOMES FROM PHASE III MANEUVER TRIAL OF PIMICOTINIB ... | 1 | HKEx | ||
| 29.09.25 | ABBISKO-B (02256): GRANT OF OPTIONS UNDER THE POST-IPO SHARE OPTION SCHEME AND GRANT OF RSUS UNDER THE 2019 SHARE INCENTIVE PLAN | 1 | HKEx | ||
| 15.09.25 | ABBISKO-B (02256): 2025 INTERIM REPORT | 3 | HKEx | ||
| 28.08.25 | ABBISKO-B (02256): DISCLOSEABLE TRANSACTION - SUBSCRIPTIONS OF WEALTH MANAGEMENT PRODUCTS | - | HKEx | ||
| 27.08.25 | ABBISKO-B (02256): NEXT DAY DISCLOSURE RETURNS | - | HKEx | ||
| 20.08.25 | ABBISKO-B (02256): VOLUNTARY ANNOUNCEMENT - IND CLEARANCE FROM THE CDE FOR ABBISKO THERAPEUTICS' ORAL PD-L1 INHIBITOR ABSK043 IN COMBINATION WITH KRAS ... | 1 | HKEx | ||
| 20.08.25 | ABBISKO-B (02256): NEXT DAY DISCLOSURE RETURNS | 2 | HKEx |
1 2 Weiter >>
22 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| PRAXIS PRECISION MEDICINES | 319,57 | +4,56 % | Praxis Precision Medicines, Inc. Announces Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | ||
| QIAGEN | 43,170 | -0,70 % | Ionos, Kontron, Lanxess, Qiagen, Redcare Pharmacy, Siltronic, TeamViewer: Neue Aktien-Positionen der Short-Seller | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| AVIDITY BIOSCIENCES | 72,90 | +0,11 % | Avidity Biosciences, Inc. - 8-K, Current Report | ||
| TARSUS PHARMACEUTICALS | 64,62 | +4,24 % | Tarsus Pharmaceuticals, Inc: Tarsus Reports Third Quarter 2025 Financial Results and Recent Business Achievements | Delivered quarterly XDEMVY® net sales of approximately $119 million, up approximately 147% year-over-year Weekly multi-patient prescribers grew approximately 30% in the third quarter underscoring... ► Artikel lesen | |
| SUMMIT THERAPEUTICS | 14,990 | +8,23 % | H.C. Wainwright reiterates Buy rating on Summit Therapeutics stock | ||
| ERASCA | 12,290 | +3,45 % | Erasca, Inc.: Erasca Announces Pricing of Upsized Public Offering of Common Stock | SAN DIEGO, Jan. 21, 2026 (GLOBE NEWSWIRE) -- Erasca, Inc. (Nasdaq: ERAS), a clinical-stage precision oncology company singularly focused on discovering, developing, and commercializing therapies for... ► Artikel lesen | |
| AGOMAB THERAPEUTICS | 14,650 | 0,00 % | AgomAb Therapeutics N.V.: Agomab Announces Pricing of Initial Public Offering | ANTWERP, Belgium, February 5, 2026 (GLOBE NEWSWIRE) - Agomab Therapeutics NV (Nasdaq: AGMB) ("Agomab"), a clinical-stage biopharmaceutical company focused on developing novel disease-modifying therapies... ► Artikel lesen | |
| RECURSION PHARMACEUTICALS | 3,960 | +11,24 % | Recursion Pharmaceuticals, Inc.: Positive Phase 1b/2 Results from Ongoing REC-4881 TUPELO Trial Demonstrate Rapid and Durable Reductions in Polyp Burden in Familial Adenomatous Polyposis (FAP) at 25 Weeks | REC-4881 (4 mg QD) achieved rapid clinical activity, with 75% of evaluable patients showing reductions in total polyp burden and a 43% median reduction after 12 weeks of treatment (n=12)After 12 weeks... ► Artikel lesen | |
| APOGEE THERAPEUTICS | 62,19 | +0,10 % | Jefferies lowers Apogee Therapeutics stock price target on OX40 concerns | ||
| IMMUNOVANT | 27,040 | +12,39 % | Immunovant Inc.: Immunovant Provides Corporate Updates and Reports Financial Results for the Third Quarter Ended December 31, 2025 | IMVT-1402 potentially registrational trial in difficult-to-treat rheumatoid arthritis (D2T RA) fully enrolled, with topline data expected in the second half of calendar year 2026; topline data from... ► Artikel lesen | |
| STRUCTURE THERAPEUTICS | 74,96 | +2,39 % | Structure Therapeutics Inc.: Structure Therapeutics Announces Initiation of Phase 1 Clinical Study of Oral Small Molecule Amylin Receptor Agonist ACCG-2671 for the Treatment of Obesity | SAN FRANCISCO, Dec. 17, 2025 (GLOBE NEWSWIRE) -- Structure Therapeutics Inc. (NASDAQ: GPCR), a clinical-stage global biopharmaceutical company developing novel oral small molecule therapeutics for... ► Artikel lesen | |
| MINERALYS THERAPEUTICS | 29,770 | +5,53 % | Mineralys Therapeutics, Inc.: Mineralys Therapeutics' Phase 3 Launch-HTN Trial of Lorundrostat Recognized in Inaugural Journal of the American Medical Association (JAMA) "Research of the Year" Roundup | - Launch-HTN, the largest trial of an aldosterone synthase inhibitor conducted among participants with uncontrolled or treatment-resistant hypertension, was one of nine studies selected as most impactful... ► Artikel lesen | |
| SPYGLASS PHARMA | 26,400 | 0,00 % | SpyGlass Pharma Prices IPO At $16 Per Share | WASHINGTON (dpa-AFX) - SpyGlass Pharma, Inc. (SGP), a late-stage biopharmaceutical company, announced the pricing of its initial public offering of 9.38 million shares at $16 per share.The company... ► Artikel lesen | |
| LIMINATUS PHARMA | 1,840 | -10,24 % | Liminatus Pharma, Inc. - 8-K, Current Report | ||
| DAY ONE BIOPHARMACEUTICALS | 11,410 | +8,87 % | Day One Biopharmaceuticals, Inc.: Day One Announces Preliminary 2025 OJEMDA Net Product Revenue And Provides 2026 Net Product Revenue Guidance | Preliminary 2025 net product revenue of $155.4 million, representing 172% year-over-year growth; OJEMDA 2026 U.S. net product revenue projected to be $225 - $250 million Company to present on corporate... ► Artikel lesen |
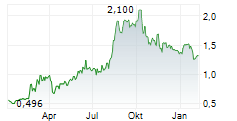
ABBISKO CAYMAN LTD gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von -0,020 (-1,52 %) liegt der Kurs bei 1,300 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ABBISKO CAYMAN LTD finden Sie auf Wallstreet Online.