- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 21.03. | ADIAL PHARMACEUTICALS, INC. - 8-K, Current Report | 1 | SEC Filings | ||
| 05.03. | Adial Pharmaceuticals reports FY results | 4 | Seeking Alpha | ||
| 04.03. | Rodman & Renshaw maintains Adial stock Buy rating, $8 target | 5 | Investing.com | ||
| 04.03. | Adial Pharmaceuticals, Inc: Adial Pharmaceuticals Reports 2024 Fiscal Year Financial Results and Provides Business Update | 204 | GlobeNewswire (Europe) | GLEN ALLEN, Va., March 04, 2025 (GLOBE NEWSWIRE) -- Adial Pharmaceuticals, Inc. (NASDAQ: ADIL) ("Adial" or the "Company"), a clinical-stage biopharmaceutical company focused on developing therapies... ► Artikel lesen | |
| 04.03. | ADIAL PHARMACEUTICALS, INC. - 8-K, Current Report | - | SEC Filings | ||
| ADIAL PHARMACEUTICALS Aktie jetzt für 0€ handeln | |||||
| 04.03. | ADIAL PHARMACEUTICALS, INC. - 10-K, Annual Report | - | SEC Filings | ||
| 25.02. | Adial erhält FDA-Zustimmung für Phase-3-Medikamentenstrategie | 4 | Investing.com Deutsch | ||
| 25.02. | Adial gets FDA nod for Phase 3 drug strategy | 4 | Investing.com | ||
| 25.02. | Adial Pharmaceuticals, Inc: Adial Pharmaceuticals Receives Positive Response from FDA Meeting Regarding Proposed In Vitro Bridging Strategy for AD04 | 144 | GlobeNewswire (Europe) | FDA feedback confirms Adial's proposed in vitro bridging strategy to the Phase 3 formulation to fulfill the bridging requirement for the 505(b)(2) regulatory registration pathway for approval Adial... ► Artikel lesen | |
| 25.02. | EXCLUSIVE: After FDA Response, Adial Starts Manufacturing Clinical Supplies For Upcoming Phase 3 Program Of Lead Candidate For Alcohol Use Disorder | 1 | Benzinga.com | ||
| 25.02. | ADIAL PHARMACEUTICALS, INC. - 8-K, Current Report | - | SEC Filings | ||
| 19.02. | Adial Pharmaceuticals secures patent for genetic addiction treatment | 2 | Investing.com | ||
| 19.02. | Adial Pharmaceuticals, Inc: Adial Pharmaceuticals Granted New U.S. Patent Expanding Coverage of its Genetic-Based Approach to Treating and Diagnosing Alcohol Use Disorder and Other Drug Dependencies | 115 | GlobeNewswire (Europe) | New Patent covers claims which focus on method for identifying genetic markers in patients with alcohol or opioid related disorders Patent expands the identification of potential treatment candidates... ► Artikel lesen | |
| 12.02. | Adial Pharmaceuticals, Inc: Adial Pharmaceuticals Awarded New U.S. Patent Covering Genotype-Specific Treatment of Opioid-Related Disorders | 179 | GlobeNewswire (Europe) | GLEN ALLEN, Va., Feb. 12, 2025 (GLOBE NEWSWIRE) -- Adial Pharmaceuticals, Inc. (NASDAQ: ADIL) ("Adial" or the "Company"), a clinical-stage biopharmaceutical company focused on developing therapies... ► Artikel lesen | |
| 12.02. | EXCLUSIVE: Adial Pharmaceuticals Secures New US Patent Covering Genotype-Specific Treatment of Opioid-Related Disorders | 3 | Benzinga.com | ||
| 29.01. | Adial completes key study for alcohol disorder drug | 1 | Investing.com | ||
| 29.01. | Adial Pharmaceuticals, Inc: Adial Pharmaceuticals Announces Positive Clinical Study Results from the AD04-103 Pharmacokinetics Study of AD04 for the Treatment of Alcohol Use Disorder | 148 | GlobeNewswire (Europe) | Completion enables the End-of-Phase 2 (EOP2) interaction with the FDA on the design of the Phase 3 program and is expected to facilitate ongoing partnership discussions Adial's formulation exhibited... ► Artikel lesen | |
| 29.01. | EXCLUSIVE: Adial Pharmaceuticals Reveals Positive Pharmacokinetics Study Results Of AD04 For Alcohol Use Disorder | 1 | Benzinga.com | ||
| 09.01. | Adial Pharmaceuticals: Interview With Cary Claiborne About The Addiction Treatment Company | 2 | pulse2.com | ||
| 08.01. | ADIAL PHARMACEUTICALS, INC. - 8-K, Current Report | - | SEC Filings |
Seite:
1 2 3 Weiter >>
48 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| EVOTEC | 6,396 | +9,90 % | EQS-News: Evotec SE stellt neue Strategie vor und gibt Ausblick für 2025 gestützt durch starkes Ergebnis im 4. Quartal 2024 | EQS-News: Evotec SE
/ Schlagwort(e): Jahresbericht
Evotec SE stellt neue Strategie vor und gibt Ausblick für 2025 gestützt durch starkes Ergebnis im 4. Quartal 2024
17.04.2025... ► Artikel lesen | |
| MEDIGENE | 0,185 | +4,23 % | Aktien KW 15 Aufatmen nach Galgenfrist. Zu früh einzusteigen? News. Aixtron. Rheinmetall. Hensoldt. Mutares. Steyr. Traton. Infineon. Redcare Pharmacy. Puma. Nordex. Medigene. SUSS MicroTec. Renk. Grammer. DHL. Tonies. Nvidia | Aktien Wochenrückblick - das schlimmste Szenario wurde diese Woche an den Börsen nicht Realität. Aber aufgeschoben heisst nicht aufgehoben. Vielleicht schreckte den Nero im Weissen Haus, dass auch US-Staatsanleihen... ► Artikel lesen | |
| CUREVAC | 2,766 | +0,36 % | CureVac-Aktie: Das wird zum Kracher! | News von Trading-Treff.de Eine erneut vernichtende Entwicklung nimmt nun die Aktie von CureVac. Das Papier hat am Donnerstag einen Abschlag in Höhe von -1,6% hinnehmen müssen. Damit ist die Aktie aktuell... ► Artikel lesen | |
| QIAGEN | 35,980 | -1,91 % | Qiagen will 2026 mit drei neuen Laborgeräten an den Markt gehen | DJ Qiagen will 2026 mit drei neuen Laborgeräten an den Markt gehen
DOW JONES--Qiagen will im nächsten Jahr drei neue Probenvorbereitungsgeräte für die Laborautomatisierung auf den Markt bringen.... ► Artikel lesen | |
| MODERNA | 21,670 | -0,32 % | Unglaubliche Prognose für Montag setzt Moderna Aktionäre unter Druck. Jetzt Sondermeldung lesen und Montag reagieren! | ||
| PAION | 0,017 | -19,81 % | Unglaubliche Prognose für Montag setzt Paion Aktionäre unter Druck. Jetzt Sondermeldung lesen und Montag reagieren! | ||
| NOVAVAX | 5,242 | -0,72 % | Novavax Aktie: Zulassungs-Chaos! | Anhaltende Ungewissheit bei der vollständigen Impfstoff-Zulassung und der Rücktritt eines hochrangigen FDA-Beamten führen zu erheblichen Kursverlusten bei Novavax. Die Aktie von Novavax steht unter... ► Artikel lesen | |
| STRYKER | 306,10 | +0,39 % | Stryker Aktie: Leichte Erholung nach Talfahrt | Trotz Jahresverlust von 12% halten Experten an Kaufempfehlungen für den Medizintechnik-Konzern fest. Hohe Bewertungskennzahlen sorgen jedoch für Skepsis. Die Stryker-Aktie zeigt heute erste Anzeichen... ► Artikel lesen | |
| BIOFRONTERA | 2,300 | -5,35 % | EQS-HV: Biofrontera AG: Bekanntmachung der Einberufung zur Hauptversammlung am 28.05.2025 in Köln mit dem Ziel der europaweiten Verbreitung gemäß §121 AktG | EQS-News: Biofrontera AG
/ Bekanntmachung der Einberufung zur Hauptversammlung
Biofrontera AG: Bekanntmachung der Einberufung zur Hauptversammlung am 28.05.2025 in Köln mit dem... ► Artikel lesen | |
| CRISPR THERAPEUTICS | 33,200 | +0,30 % | Crispr Therapeutics Aktie: Verliert ihren Kurs? | Der Austritt des operativen Geschäftsführers beim Gentherapie-Unternehmen beunruhigt Investoren und wirft Fragen zur Projektkontinuität im wettbewerbsintensiven Biotech-Sektor auf. Die Finanzmärkte... ► Artikel lesen | |
| PALATIN TECHNOLOGIES | 0,189 | -1,56 % | Palatin's Phase 2 Obesity Study Shows Encouraging Appetite Suppression Data, Stock Up In Pre-Market | WASHINGTON (dpa-AFX) - Palatin Technologies, Inc. (PTN), Thursday announced results from its BMT-801 Phase 2 obesity study, which evaluated the co-administration of melanocortin 4 receptor or... ► Artikel lesen | |
| CO.DON | 0,016 | -8,57 % | CO.DON GmbH ist Partner des EU-geförderten Projekts zur Weiterentwicklung des 3D-Bioprinting für die Geweberegeneration | Teltow (ots) - Das im Dezember 2024 gestartete EU-geförderte Projekt micro2MACRO (m2M) zielt darauf ab, die Geweberegeneration durch eine innovative Bioprinting-Plattform voranzutreiben. m2M konzentriert... ► Artikel lesen | |
| BIOXXMED | 0,350 | -12,50 % | XETR DELETION OF INSTRUMENTS FROM XETRA - 31.03.2025 | The following instruments on Xetra do have their last trading day on 31.03.2025.Die folgenden Instrumente auf Xetra haben ihren letzten Handelstag am 31.03.2025.ISIN NameDE000A4BGGE4 bioXXmed AG ► Artikel lesen | |
| ORGANOVO | 1,930 | -100,00 % | Organovo, Inc.: Organovo Provides Update on Cash and Nasdaq Continued Listing Requirements | SAN DIEGO, April 02, 2025 (GLOBE NEWSWIRE) -- Organovo Holdings, Inc. (Nasdaq: ONVO) ("Organovo" or the "Company"), a clinical stage biotechnology company focused on developing novel treatment approaches... ► Artikel lesen | |
| OCUGEN | 0,593 | -1,36 % | Ocugen Aktie: Attraktive Möglichkeiten entdeckt! | Trotz Jahresrückgangs zeigt das US-Biotechnologieunternehmen eine beeindruckende 7-Tage-Performance mit positiven Analystenprognosen bei der Gentherapie-Entwicklung. Die Aktie des US-Biotechnologieunternehmens... ► Artikel lesen |
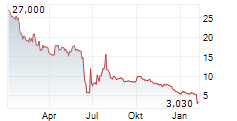
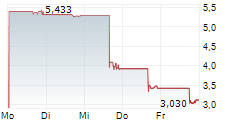
ADIAL PHARMACEUTICALS INC gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von +0,033 (+4,81 %) liegt der Kurs bei 0,719 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und .
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ADIAL PHARMACEUTICALS INC finden Sie auf Wallstreet Online.