| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
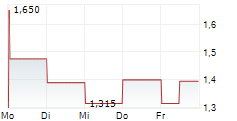
| 1,385 | 1,455 | 06.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 26.01. | Aelis Farma Announces Its 2026 Financial Calendar | 179 | Business Wire | Regulatory News:
Aelis Farma (ISIN: FR0014007ZB4 Ticker: AELIS, PEA-PME eligible), a clinical-stage biopharmaceutical company specializing in the development of treatments for brain and peripheral... ► Artikel lesen | |
| 12.01. | Aelis Farma Receives a Positive Opinion From EMA Pediatric Committee on the Pediatric Investigation Plan for AEF0217 in Down Syndrome | 222 | Business Wire | The EMA's Pediatric Committee (PDCO) has delivered a favorable consensus opinion on the Pediatric Investigational Plan (PIP) of Aelis Farma's first-in-class drug candidate AEF0217 for the treatment... ► Artikel lesen | |
| 02.12.25 | Aelis Farma Obtains Regulatory Approval of the Phase 2B Trial With AEF0217 in People With Down Syndrome | 372 | Business Wire | AEF0217 is a first-in-class CB1 receptor Signalling Specific inhibitor (CB1-SSi) developed as a potential first pharmacological treatment for impairments in adaptive behaviours and cognition... ► Artikel lesen | |
| 25.09.25 | Aelis Farma: Availability of the 2025 Half-Year Financial Report | 319 | Business Wire | Regulatory News:
Aelis Farma (ISIN: FR0014007ZB4 Ticker: AELIS), a clinical-stage biopharmaceutical company specialising in the development of treatments for brain and peripheral diseases involving... ► Artikel lesen | |
| 22.09.25 | Aelis Farma Reports 2025 Half-Year Financial Results and Presents Progress and Development Outlook | 490 | Business Wire | The first half of 2025 was marked by: The publication of the final analysis of the pioneering Phase 2b clinical trial in cannabis use disorders (CUD) with the CB1-SSi AEF0117, which further... ► Artikel lesen | |
| 24.06.25 | Aelis Farma: Availability of the Description of the Share Buyback Program | 390 | Business Wire | Regulatory News:
Aelis Farma (ISIN: FR0014007ZB4 Ticker: AELIS), a clinical-stage biopharmaceutical company specializing in the development of treatments for brain and peripheral diseases involving... ► Artikel lesen | |
| AELIS FARMA Aktie jetzt für 0€ handeln | |||||
| 27.05.25 | Results of Aelis Farma Combined General Meeting of May 27, 2025 | 418 | Business Wire | Regulatory News:
Aelis Farma (ISIN: FR0014007ZB4 Ticker: AELIS), clinical-stage biopharmaceutical company specializing in the development of treatments for brain and peripheral diseases involving... ► Artikel lesen | |
| 06.05.25 | Aelis Farma: Combined General Meeting of May 27, 2025: Availability of Preparatory Documents and Participation and Voting Procedures | 421 | Business Wire | Regulatory News:
Aelis Farma (ISIN: FR0014007ZB4 Ticker: AELIS), a clinical-stage biopharmaceutical company specializing in the development of treatments for brain and peripheral diseases involving... ► Artikel lesen | |
| 29.04.25 | Aelis Farma: Availability of the 2024 Universal Registration Document | 342 | Business Wire | Regulatory News:
Aelis Farma (ISIN: FR0014007ZB4 Ticker: AELIS), a clinical-stage biopharmaceutical company specializing in the development of treatments for brain and peripheral diseases involving... ► Artikel lesen | |
| 01.04.25 | Aelis Farma Reports Its 2024 Annual Financial Results and Confirms Its 2025 Outlook | 536 | Business Wire | 2024 has been marked by several significant events: The achievement of key milestones for the Company's two drug candidates:
AEF0117: announcement of the results of the Phase 2b clinical... ► Artikel lesen | |
| 26.03.25 | Aelis Farma Announces the Final Analysis of the Landmark Phase 2B Clinical Trial in Cannabis Use Disorder (CUD) with the CB1-SSi AEF0117 | 556 | Business Wire | The purpose of this pioneering Phase 2B trial was to show that AEF0117 reduces cannabis use and to determine the endpoints and optimal dosage to be used in future studies. AEF0117 is the first... ► Artikel lesen | |
| 17.02.25 | Aelis Farma Announces Its 2025 Financial Calendar | 375 | Business Wire | Regulatory News:
Aelis Farma (ISIN: FR0014007ZB4 Ticker: AELIS), a clinical-stage biopharmaceutical company specializing in the development of treatments for brain and peripheral diseases involving... ► Artikel lesen |
12 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| PRAXIS PRECISION MEDICINES | 319,57 | +4,56 % | Praxis Precision Medicines, Inc. Announces Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | ||
| QIAGEN | 43,170 | -0,70 % | Qiagen übertrifft Markterwartungen im vierten Quartal | DJ Qiagen übertrifft Markterwartungen im vierten Quartal
DOW JONES--Qiagen hat im Schlussquartal 2025 den Umsatz gesteigert, der operative Gewinn ging gegenüber dem Vorjahr zurück. 2026 rechnet... ► Artikel lesen | |
| AVIDITY BIOSCIENCES | 72,90 | +0,11 % | Avidity Biosciences, Inc. - 8-K, Current Report | ||
| TARSUS PHARMACEUTICALS | 64,62 | +4,24 % | Tarsus Pharmaceuticals, Inc: Tarsus Reports Third Quarter 2025 Financial Results and Recent Business Achievements | Delivered quarterly XDEMVY® net sales of approximately $119 million, up approximately 147% year-over-year Weekly multi-patient prescribers grew approximately 30% in the third quarter underscoring... ► Artikel lesen | |
| SUMMIT THERAPEUTICS | 14,990 | +8,23 % | H.C. Wainwright reiterates Buy rating on Summit Therapeutics stock | ||
| ERASCA | 12,290 | +3,45 % | Erasca, Inc.: Erasca Announces Pricing of Upsized Public Offering of Common Stock | SAN DIEGO, Jan. 21, 2026 (GLOBE NEWSWIRE) -- Erasca, Inc. (Nasdaq: ERAS), a clinical-stage precision oncology company singularly focused on discovering, developing, and commercializing therapies for... ► Artikel lesen | |
| RECURSION PHARMACEUTICALS | 3,960 | +11,24 % | Recursion Pharmaceuticals, Inc.: Positive Phase 1b/2 Results from Ongoing REC-4881 TUPELO Trial Demonstrate Rapid and Durable Reductions in Polyp Burden in Familial Adenomatous Polyposis (FAP) at 25 Weeks | REC-4881 (4 mg QD) achieved rapid clinical activity, with 75% of evaluable patients showing reductions in total polyp burden and a 43% median reduction after 12 weeks of treatment (n=12)After 12 weeks... ► Artikel lesen | |
| APOGEE THERAPEUTICS | 62,19 | +0,10 % | Jefferies lowers Apogee Therapeutics stock price target on OX40 concerns | ||
| IMMUNOVANT | 27,040 | +12,39 % | Immunovant Inc.: Immunovant Provides Corporate Updates and Reports Financial Results for the Third Quarter Ended December 31, 2025 | IMVT-1402 potentially registrational trial in difficult-to-treat rheumatoid arthritis (D2T RA) fully enrolled, with topline data expected in the second half of calendar year 2026; topline data from... ► Artikel lesen | |
| STRUCTURE THERAPEUTICS | 74,96 | +2,39 % | Structure Therapeutics Inc.: Structure Therapeutics Announces Initiation of Phase 1 Clinical Study of Oral Small Molecule Amylin Receptor Agonist ACCG-2671 for the Treatment of Obesity | SAN FRANCISCO, Dec. 17, 2025 (GLOBE NEWSWIRE) -- Structure Therapeutics Inc. (NASDAQ: GPCR), a clinical-stage global biopharmaceutical company developing novel oral small molecule therapeutics for... ► Artikel lesen | |
| MINERALYS THERAPEUTICS | 29,770 | +5,53 % | Mineralys Therapeutics, Inc.: Mineralys Therapeutics' Phase 3 Launch-HTN Trial of Lorundrostat Recognized in Inaugural Journal of the American Medical Association (JAMA) "Research of the Year" Roundup | - Launch-HTN, the largest trial of an aldosterone synthase inhibitor conducted among participants with uncontrolled or treatment-resistant hypertension, was one of nine studies selected as most impactful... ► Artikel lesen | |
| LIMINATUS PHARMA | 1,840 | -10,24 % | Liminatus Pharma, Inc. - 8-K, Current Report | ||
| DAY ONE BIOPHARMACEUTICALS | 11,410 | +8,87 % | Day One Biopharmaceuticals, Inc.: Day One Announces Preliminary 2025 OJEMDA Net Product Revenue And Provides 2026 Net Product Revenue Guidance | Preliminary 2025 net product revenue of $155.4 million, representing 172% year-over-year growth; OJEMDA 2026 U.S. net product revenue projected to be $225 - $250 million Company to present on corporate... ► Artikel lesen | |
| AMYLYX PHARMACEUTICALS | 13,980 | +7,62 % | Gubra A/S: Amylyx Pharmaceuticals Announces Nomination of AMX0318 as a Novel, Long-Acting GLP-1 Receptor Antagonist Development Candidate, Identified in Collaboration with Gubra | AMX0318 selected as development candidate after meeting key criteria, demonstrating a robust chemical stability profile, strong in vitro potency, evidence of in vivo efficacy and tolerability, high... ► Artikel lesen | |
| OLEMA PHARMACEUTICALS | 25,360 | +1,40 % | Olema Oncology Reports Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) |
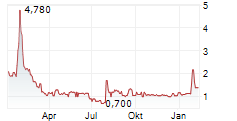
AELIS FARMA SAS gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von -0,005 (-0,36 %) liegt der Kurs bei 1,395 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu AELIS FARMA SAS finden Sie auf Wallstreet Online.