| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
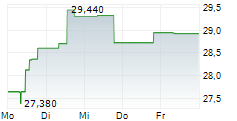
| 19,570 | 19,590 | 11:26 | |
| 19,570 | 19,590 | 11:26 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mi | ALK Abello: ITULAZAX tree pollen tablet approved for treatment of children in Europe | 70 | GlobeNewswire (Europe) | ALK (ALKB.DC / OMX: ALK B) today announced that its European regulatory filing for ITULAZAX® (tree pollen sublingual allergy immunotherapy tablet) for treatment of young children and adolescents aged... ► Artikel lesen | |
| 03.04. | ALK Abello: Grant of share-based long-term incentive instruments to members of the Board of Management and key employees | 1 | GlobeNewswire (USA) | ||
| 13.03. | ALK Abello: Annual General Meeting in ALK-Abelló A/S held on 13 March 2025 | 3 | GlobeNewswire (USA) | ||
| 07.03. | ALK Abello: ACARIZAX approved in Canada for treatment of young children | 260 | GlobeNewswire (Europe) | ALK (ALKB:DC / OMX: ALK B) today announced that Health Canada has approved ALK's ACARIZAX® tablet for use in children, aged five to 11, with house dust mite (HDM) allergy. ACARIZAX® is now indicated... ► Artikel lesen | |
| 04.03. | ALK Abello: Report on transactions with ALK-Abelló A/S B shares and associated securities by managerial staff | 3 | GlobeNewswire (USA) | ||
| 27.02. | ALK Abello: FDA approves ODACTRA for the treatment of house dust mite allergy in young children | 292 | GlobeNewswire (Europe) | ALK (ALKB:DC / OMX: ALK B) today announced that the US Food and Drug Administration (FDA) has approved ALK's ODACTRA® tablet for use in young children with house dust mite (HDM) allergy. ODACTRA®... ► Artikel lesen | |
| 21.02. | ALK Abello: Report on transactions with ALK-Abelló A/S B shares and associated securities by managerial staff | 3 | GlobeNewswire (USA) | ||
| ALK-ABELLO Aktie jetzt für 0€ handeln | |||||
| 19.02. | ALK-Abelló projects 9%-13% revenue growth for 2025 driven by global tablet expansion | 1 | Seeking Alpha | ||
| 19.02. | ALK Abello: Annual General Meeting in ALK-Abelló A/S on 13 March 2025 | 2 | GlobeNewswire (USA) | ||
| 19.02. | ALK-Abello shares rise following 4Q earnings report | 5 | Investing.com | ||
| 19.02. | ALK-Abelló A/S reports FY results | 3 | Seeking Alpha | ||
| 19.02. | ALK Abello: Annual report 2024: ALK delivers 15% sales growth with profits up 65% | 254 | GlobeNewswire (Europe) | ALK's (ALKB:DC / OMX: ALK B / AKBLF) Board of Directors has approved the 2024 annual report. Following a robust performance in Q4, full-year results were in line with the latest outlook and represent... ► Artikel lesen | |
| 12.02. | ALK Abello: Invitation to the presentation of ALK's annual report 2024 on Wednesday, 19 February 2025 | 4 | GlobeNewswire (USA) | ||
| 31.01. | ALK-Abello stock jumps as Jefferies upgrades to 'buy' on growth prospects | 16 | Investing.com | ||
| 30.01. | ALK Abello: ALK's house dust mite tablet (ACARIZAX) now recommended by NICE for use in the UK health system | 351 | GlobeNewswire (Europe) | ALK (ALKB:DC / OMX: ALK B / AKBLF) today announced that the National Institute for Health and Care Excellence (NICE) has recommended the use of ACARIZAX® for the treatment of persistent, moderate... ► Artikel lesen | |
| 06.01. | ARS Pharmaceuticals, Inc.: ARS Pharmaceuticals Files for Approval of neffy in Canada and the United Kingdom on Behalf of Licensing Partner ALK-Abelló A/S | 4 | GlobeNewswire (USA) | ||
| 20.12.24 | ALK Abello: ALK - Financial calendar for the 2025 financial year | 2 | GlobeNewswire (USA) | ||
| 18.12.24 | ALK Abello: Positive results advance peanut tablet to phase II development | 197 | GlobeNewswire (Europe) | ALK's peanut tablet shown to be safe and tolerable across multiple doses.Development now advances to phase II for dose finding and efficacy. First patients in phase II to receive first doses imminently.... ► Artikel lesen | |
| 12.12.24 | ALK Abello: ACARIZAX approved in Europe for treatment of young children | 4 | GlobeNewswire (USA) | ||
| 27.11.24 | ALK Abello: ALK's pivotal phase 3 trial in children published in reputable scientific journal | 1 | GlobeNewswire (USA) |
Seite:
1 2 Weiter >>
32 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 22,315 | +0,95 % | Bayer-Aktie: Kurs nur geringfügig im Minus (20,885 €) | Wenig Kursbewegung derzeit bei der Aktie von Bayer . Das Wertpapier notiert gegenwärtig bei 20,89 Euro. Kaum verändert im Vergleich zu der letzten Notierung des vorigen Handelstages zeigt sich aktuell... ► Artikel lesen | |
| NOVO NORDISK | 54,10 | -0,26 % | Blockbuster oder Übernahmefieber? Warum Novo Nordisk, Defence Therapeutics und Pfizer jetzt alle Blicke auf sich ziehen | Die Biotech- und Pharmabranche gleicht einem Schachbrett der Milliarden! Ein einziger Zug - ob Übernahme oder Zulassung - kann Aktienkurse in Stunden verdoppeln oder ganze Märkte neu ordnen. Während... ► Artikel lesen | |
| PFIZER | 19,708 | -0,27 % | Ihre wichtigsten Termine: Alle Blicke auf: Intel, Pepsico, Pfizer, Boeing, Nestle, Asos, Johnson & Johnson | © Foto: Intel CorporationGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| NOVARTIS | 97,33 | +0,32 % | Novartis: Das Allzeithoch ist in Schlagdistanz! | ||
| MERCK KGAA | 119,70 | -0,75 % | Aktienmarkt: Kurs der Aktie von Merck im Plus (69,1413 €) | Im Plus liegt aktuell die Merck-Aktie . Zuletzt zahlten Investoren für das Papier 78,55 US-Dollar. Ein Preisanstieg von 2,73 Prozent steht gegenwärtig für das Wertpapier von Merck zu Buche. Die Aktie... ► Artikel lesen | |
| GILEAD SCIENCES | 93,41 | -0,50 % | Interessante Einstiege finden - BioNTech, BioNxt Solutions, Gilead Sciences, Merck & Co. | Die aggressive Zollpolitik der US-Administration unter US-Präsident Trump hat zu Umschichtungsprozessen bei den Anlegern geführt. So zogen europäische ETF-Anleger über eine Milliarde Euro aus US-Aktien-ETFs... ► Artikel lesen | |
| AURORA CANNABIS | 3,820 | -2,30 % | Aurora Cannabis Aktie: Ein neuer Lichtblick! | Der kanadische Cannabisproduzent positioniert sich durch internationale Markterschließung, technologische Innovation und solide Finanzlage für langfristiges Wachstum. Aurora Cannabis zieht weiterhin... ► Artikel lesen | |
| SANOFI | 92,37 | -1,10 % | Aktienmarkt: Kurs der Sanofi SA-Aktie im Minus (89,69 €) | Die Sanofi SA-Aktie notiert heute etwas leichter. Das Papier kostete zuletzt 89,69 Euro. Die Aktie von Sanofi SA verzeichnet derzeit einen Abschlag von 0,80 Prozent. Sie hat sich um 72 Cent gegenüber... ► Artikel lesen | |
| GSK | 16,330 | +0,74 % | GSK erhält in den USA Empfehlungen für Meningitis- und RSV-Impfstoffe | DJ GSK erhält in den USA Empfehlungen für Meningitis- und RSV-Impfstoffe
Von Cristina Gallardo
DOW JONES--Der britische Pharmakonzern GSK hat von einem Beratungsgremium des US-Gesundheitsministeriums... ► Artikel lesen | |
| ABBVIE | 156,00 | -0,26 % | ABBVIE mit Prognosesenkung | Der Biopharmakonzern rechnet mit Zusatzkosten in Höhe von 248 Mio. $ im Zusammenhang mit Übernahmen, darunter Meilensteinzahlungen sowie F&E-Aufwendungen. Hintergrund ist der strategische Fokus auf... ► Artikel lesen | |
| ROCHE | 274,70 | -0,20 % | JPMORGAN stuft ROCHE HOLDINGS AG auf 'Underweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für Roche auf "Underweight" mit einem Kursziel von 230 Franken belassen. Analyst Richard Vosser bezog sich am Donnerstag auf Studiendaten... ► Artikel lesen | |
| CANOPY GROWTH | 1,076 | -0,37 % | Canopy Growth Aktie: Rechtsstreit und Finanzprobleme | Canopy Growth sieht sich mit einer Sammelklage konfrontiert und meldet rückläufige Umsätze. Die geplante Kapitalerhöhung könnte die Aktie weiter belasten. Klagewelle rollt anAnzeigeSollten Anleger sofort... ► Artikel lesen | |
| STADA ARZNEIMITTEL | - | - | MIDDAY BRIEFING - Unternehmen und Märkte: CONTINENTAL, DAIMLER TRUCK, PORSCHE, HUGO BOSS, AUMANN, BAYWA, STADA, BYD, OMV, STELLANTIS | DJ MIDDAY BRIEFING - Unternehmen und Märkte
Der Markt-Überblick am Mittag, zusammengestellt von Dow Jones Newswires:
+++++ AKTIENMÄRKTE (13:18) +++++
zuletzt +/-... ► Artikel lesen | |
| ELI LILLY | 724,60 | +0,12 % | Nachfrage nach Eli Lilly verhilft S&P 500 in die Gewinnzone (5.322 Pkt.) | Leichte Kursgewinne verzeichnen zur Stunde die Papiere im S&P 500-Index . Das Aktienbarometer steigt um 0,88 Prozent auf 5.322 Punkte. Die Bullen haben gegenwärtig an der Börse überwiegend das Sagen.... ► Artikel lesen | |
| MERCK & CO | 70,00 | +0,86 % | Merck Stock Slumps Ahead Of Q1 Earnings: Can Bulls Find Relief In Buying Pressure? |
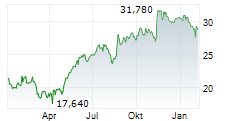
ALK-ABELLO A/S gehört der Branche Pharma an. Die relative Kursveränderung beträgt aktuell -0,56 % bei einem Aktienkurs von 19,580 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und .
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ALK-ABELLO A/S finden Sie auf Wallstreet Online.