| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 4,250 | 4,390 | 13:05 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Do | Alvotech higher on positive data backing biosimilar to Takeda's Entyvio | 6 | Seeking Alpha | ||
| Do | Alvotech - 6-K, Report of foreign issuer | 2 | SEC Filings | ||
| Do | Alvotech Announces Positive Top-Line Results from Pivotal Pharmacokinetic Study for Proposed Biosimilar to Entyvio | 164 | GlobeNewswire (Europe) | The study, which assessed the pharmacokinetics, safety, tolerability and immunogenicity of AVT80 compared to Entyvio in healthy adult participants, met all its primary endpoints REYKJAVIK, Iceland... ► Artikel lesen | |
| Mo | Alvotech Enters Supply, Commercialization Deal With Sandoz Covering Biosimilar Candidates | 16 | RTTNews | ||
| ALVOTECH Aktie jetzt für 0€ handeln | |||||
| Mo | Alvotech enters supply and commercialization agreements for Canada and Australia & New Zealand covering multiple biosimilar candidates | 135 | GlobeNewswire (Europe) | REYKJAVIK, Iceland, Feb. 02, 2026 (GLOBE NEWSWIRE) -- Alvotech (NASDAQ: ALVO), a global biotechnology company specializing in the development and manufacture of biosimilar medicines for patients worldwide... ► Artikel lesen | |
| 29.01. | Alvotech - 6-K, Report of foreign issuer | 4 | SEC Filings | ||
| 29.01. | Alvotech resolves global patent disputes with Regeneron and Bayer over Eylea biosimilar | 53 | Seeking Alpha | ||
| 29.01. | Alvotech Secures Settlement Agreement in Global Markets for its Biosimilar to Eylea 2mg | 217 | GlobeNewswire (Europe) | REYKJAVIK, Iceland, Jan. 29, 2026 (GLOBE NEWSWIRE) -- Alvotech (NASDAQ: ALVO), a global biotechnology company specializing in the development and manufacture of biosimilar medicines for patients worldwide... ► Artikel lesen | |
| 06.01. | Alvotech: Transactions of Managers and Closely Associated Persons | 18 | GlobeNewswire (USA) | ||
| 06.01. | Alvotech announces CEO transition | 10 | Seeking Alpha | ||
| 06.01. | Alvotech Appoints Lisa Graver As CEO, Rbert Wessman To Continue As Executive Chairman | 3 | RTTNews | ||
| 06.01. | Alvotech names Lisa Graver as new CEO as founder steps back | 4 | Investing.com | ||
| 06.01. | Alvotech announces planned CEO succession and leadership transition | 386 | GlobeNewswire (Europe) | R- bert Wessman to continue serving as Executive Chairman in full-time capacityLisa Graver appointed Chief Executive Officer REYKJAVIK, ICELAND (January 6, 2026) - Alvotech (NASDAQ: ALVO), a global... ► Artikel lesen | |
| 06.01. | Alvotech Secures $100 Million Term Loan Facility Led By GoldenTree | 13 | pulse2.com | ||
| 02.01. | Alvotech - 6-K, Report of foreign issuer | 7 | SEC Filings | ||
| 31.12.25 | Alvotech Secures Term Loan Facility of USD 100 Million | 6 | GlobeNewswire (USA) | ||
| 29.12.25 | Alvotech: Changes in company's own shares | 13 | GlobeNewswire (USA) | ||
| 23.12.25 | Alvotech - 6-K, Report of foreign issuer | 3 | SEC Filings | ||
| 22.12.25 | Alvotech's Financial Calendar for 2026 | 17 | GlobeNewswire (USA) | ||
| 22.12.25 | Alvotech announces European launch of biosimilar to Simponi | 14 | Seeking Alpha |
1 2 3 4 5 Weiter >>
123 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 90,40 | +0,17 % | Startschuss! BioTech-Sektor profitiert von Rotation! Augen auf bei Evotec, Bayer, Vidac Pharma and BioNTech | Mit positiver Grundstimmung hat die Börse das Jahr 2026 eingeläutet. Dass Renditechancen nicht allein im Technologiesektor liegen, bewies zuletzt der Minen- und Rohstoffbereich, wo etliche Titel Kursverdopplungen... ► Artikel lesen | |
| BB BIOTECH | 50,60 | -0,78 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| PAION | 0,200 | +8,11 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WIRD GEPRUEFT | Fuer folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wird ein Mistrade-Antrag geprueft:ISIN Datum Zeit Volumen Preis Fair Value (AS)DE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 3,986 | +0,61 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| EPIGENOMICS | 0,870 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| BIOFRONTERA | 2,420 | -1,22 % | PTA-news: Biofrontera AG: Biofrontera berichtet über die Ergebnisse der ersten neun Monate des Jahres 2025 | DJ PTA-News: Biofrontera AG: BIOFRONTERA BERICHTET ÜBER DIE ERGEBNISSE DER ERSTEN NEUN MONATE DES JAHRES 2025
Unternehmensmitteilung für den Kapitalmarkt
Biofrontera AG: BIOFRONTERA... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,910 | 0,00 % | HEIDELBERG PHARMA AG zeigt klare Stärke im Trend | ||
| CRISPR THERAPEUTICS | 41,200 | -0,48 % | Morningstar, Inc.: Morningstar Completes Acquisition of CRSP and Extends Relationship with Vanguard | The CRSP integration unites two trusted sources of market insight, reinforcing a shared commitment to transparency, quality, and investor-focused solutions and solidifying Morningstar's position... ► Artikel lesen | |
| PALATIN TECHNOLOGIES | 15,510 | -3,21 % | Palatin Technologies, Inc.: Palatin Reports First Quarter Fiscal Year 2026 Financial Results and Provides Corporate Update | Significant progress on multiple fronts - obesity pipeline advancing towards the clinic, out-licensing collaboration, strengthened balance sheet, and reinstatement... ► Artikel lesen | |
| BIOXXMED | 1,328 | 0,00 % | XFRA NEW INSTRUMENTS AVAILABLE ON 14.01.2026 | The following instruments on XETRA do have their first trading 14.01.2026 Die folgenden Instrumente in XETRA haben ihren ersten Handelstag 14.01.2026
Aktien
1 MHY3130D1013 Heidmar Maritime Hldgs.... ► Artikel lesen | |
| VIVOSIM LABS | 1,220 | -100,00 % | Morning Market Movers: TechCreate, VivoSim Labs, La Rosa Holdings, Kaixin Holdings See Big Swings | BEIJING (dpa-AFX) - At 7:40 a.m. ET on Friday, premarket trading is seeing notable activity in several stocks, with early price movements signaling potential opportunities before the opening... ► Artikel lesen | |
| INOVIO PHARMACEUTICALS | 1,410 | +0,71 % | INOVIO Pharmaceuticals, Inc.: FDA Accepts for Review INOVIO's BLA for INO-3107 for the Treatment of Adults with Recurrent Respiratory Papillomatosis (RRP) | PLYMOUTH MEETING, Pa., Dec. 29, 2025 /PRNewswire/ -- INOVIO (NASDAQ: INO), a biotechnology company focused on developing and commercializing DNA medicines to help... ► Artikel lesen | |
| OCUGEN | 1,150 | +1,05 % | Ocugen Announces Positive Preliminary Phase 2 Data from OCU410 Modifier Gene Therapy for Geographic Atrophy Secondary to Dry Age-Related Macular Degeneration | Phase 2 (~50% of patients evaluated to date at 12 months) shows 46% lesion growth reduction vs. controlThere are no OCU410-related serious adverse events reported across the Phase 1 and Phase 2 clinical... ► Artikel lesen | |
| INFLARX | 0,730 | -2,54 % | Weekly Buzz: ARS Pharma's Neffy Goes East, SLS Advances, A Signal Of Hope For IFRX? | LONDON (dpa-AFX) - Despite the typical year-end slowdown, the week saw a steady stream of high-impact news, highlighted by global approvals, clinical trial readouts, new product launches, and... ► Artikel lesen | |
| NOVONESIS | 51,50 | +0,04 % | Novonesis (Novozymes A/S): Novonesis delivered 8% organic sales growth in the first nine months of 2025 |
Novonesis delivered 8% organic sales growth in the first nine months of 2025 and narrowed full-year outlook upwards. COPENHAGEN, Denmark - November 6, 2025. Novonesis delivered 8% organic sales growth... ► Artikel lesen |
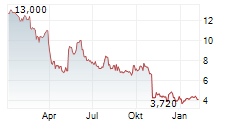
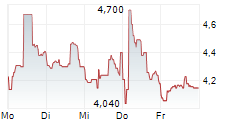
ALVOTECH gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 4,260 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ALVOTECH finden Sie auf Wallstreet Online.