| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
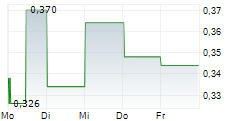
| 0,346 | 0,396 | 13:04 | |
| 0,346 | 0,396 | 06.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| ANTENGENE Aktie jetzt für 0€ handeln | |||||
| 17.12.25 | Antengene Secures Malaysian Approval For XPOVIO In Relapsed/Refractory DLBCL | - | RTTNews | ||
| 16.12.25 | ANTENGENE-B (06996): VOLUNTARY ANNOUNCEMENT XPOVIO APPROVED IN MALAYSIA FOR A NEW INDICATION IN DIFFUSE LARGE B-CELL LYMPHOMA | 1 | HKEx | ||
| 12.12.25 | ANTENGENE-B (06996): NOMINATION AND CORPORATE GOVERNANCE COMMITTEE - TERMS OF REFERENCE | 4 | HKEx | ||
| 03.12.25 | ANTENGENE-B (06996): VOLUNTARY ANNOUNCEMENT XPOVIO APPROVED IN HONG KONG FOR TWO ADDITIONAL INDICATIONS IN MULTIPLE MYELOMA AND DIFFUSE LARGE B-CELL LYMPHOMA | - | HKEx | ||
| 02.12.25 | Antengene: NMPA Approves IND Application For Phase Ib/II CLINCH-2 Study With ATG-022 | - | RTTNews | ||
| 02.12.25 | Antengene Corporation Limited: Antengene Announces IND Approval in China for Phase Ib/II Study of ATG-022 (CLDN18.2 ADC) in Combination with KEYTRUDA (Pembrolizumab) ± Chemotherapy | 145 | PR Newswire | SHANGHAI and HONG KONG, Dec. 2, 2025 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative, commercial-stage global biotech... ► Artikel lesen | |
| 02.12.25 | ANTENGENE-B (06996): VOLUNTARY ANNOUNCEMENT IND APPROVAL IN CHINA FOR PHASE IB/II STUDY OF ATG-022 IN COMBINATION WITH KEYTRUDA (PEMBROLIZUMAB) CHEMOTHERAPY | 2 | HKEx | ||
| 20.10.25 | Antengene Corporation Limited: Antengene Presents Latest ATG-022 Clinical Data at ESMO 2025 Demonstrating Efficacy Across All CLDN18.2 Expression Levels and Exceptional Tolerability | 474 | PR Newswire | SHANGHAI and HONG KONG, Oct. 20, 2025 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK) today announced the latest results from the ongoing Phase I/II CLINCH... ► Artikel lesen | |
| 15.10.25 | JPMorgan nimmt Antengene-Aktie mit "Overweight" in die Bewertung auf | 2 | Investing.com Deutsch | ||
| 15.10.25 | JPMorgan initiates Antengene stock coverage with Overweight rating | 1 | Investing.com | ||
| 29.09.25 | ANTENGENE-B (06996): 2025 INTERIM REPORT | 1 | HKEx | ||
| 19.09.25 | Antengene Corporation Limited: Antengene to Present Latest Preclinical Results from ATG-201 (CD19xCD3 TCE) at ACR 2025 | 357 | PR Newswire | SHANGHAI and HONG KONG, Sept. 19, 2025 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK) today announced that it will release the latest preclinical data of ATG-201... ► Artikel lesen | |
| 10.09.25 | ANTENGENE-B (06996): NEXT DAY DISCLOSURE RETURN | - | HKEx | ||
| 01.09.25 | ANTENGENE-B (06996): VOLUNTARY ANNOUNCEMENT INTENTION TO CONDUCT ON-MARKET SHARE REPURCHASE | 1 | HKEx | ||
| 25.08.25 | Antengene Corporation Limited: Antengene Announces 2025 Interim Results with Encouraging Clinical Data and Progress in TCE Platform | 407 | PR Newswire | SHANGHAI and HONG KONG, Aug. 25, 2025 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK) today announced its interim results for the period ending June 30, 2025, along... ► Artikel lesen | |
| 22.08.25 | Antengene Corporation Limited: Antengene Announces 2025 Interim Financial Results Highlighting Encouraging Data from Mid/Late-Stage Clinical Programs and Its Innovative TCE Technology Platform | 189 | PR Newswire | The Phase I/II CLINCH study of ATG-022 (CLDN18.2 antibody-drug conjugate) demonstrated promising results, showing robust clinical efficacy and a favorable safety... ► Artikel lesen | |
| 22.08.25 | ANTENGENE-B (06996): ANNOUNCEMENT OF INTERIM RESULTS FOR THE SIX MONTHS ENDED JUNE 30, 2025 | 1 | HKEx | ||
| 19.08.25 | Antengene Corporation Limited: Antengene's ATG-022 (CLDN18.2 ADC) Granted Breakthrough Therapy Designation for the Treatment of Gastric/Gastroesophageal Junction Adenocarcinoma | 331 | PR Newswire | SHANGHAI and HONG KONG, Aug. 19, 2025 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative, commercial-stage global biotech company dedicated to... ► Artikel lesen | |
| 19.08.25 | ANTENGENE-B (06996): VOLUNTARY ANNOUNCEMENT ATG-022 GRANTED BREAKTHROUGH THERAPY DESIGNATION FOR THE TREATMENT OF GASTRIC/GASTROESOPHAGEAL JUNCTION ADENOCARCINOMA | 2 | HKEx | ||
| 12.08.25 | ANTENGENE-B (06996): NOTICE OF DATE OF BOARD MEETING | 5 | HKEx |
1 2 Weiter >>
23 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 90,40 | +0,17 % | Startschuss! BioTech-Sektor profitiert von Rotation! Augen auf bei Evotec, Bayer, Vidac Pharma and BioNTech | Mit positiver Grundstimmung hat die Börse das Jahr 2026 eingeläutet. Dass Renditechancen nicht allein im Technologiesektor liegen, bewies zuletzt der Minen- und Rohstoffbereich, wo etliche Titel Kursverdopplungen... ► Artikel lesen | |
| EVOTEC | 6,148 | +0,79 % | SDAX: Evotec-Aktie schießt zweistellig nach oben | Eine Kaufempfehlung von Berenberg entfacht neuen Optimismus bei Evotec und katapultiert die Biotech-Aktie an die Indexspitze. Die Aktie der Evotec hat am Dienstagmittag ein Kursfeuerwerk gezündet und... ► Artikel lesen | |
| MEDIGENE | 0,042 | -8,26 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 43,170 | -0,70 % | Ionos, Kontron, Lanxess, Qiagen, Redcare Pharmacy, Siltronic, TeamViewer: Neue Aktien-Positionen der Short-Seller | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| MODERNA | 34,650 | -0,07 % | Arbutus jumps on ruling in patent dispute with Moderna | ||
| PAION | 0,200 | +8,11 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WIRD GEPRUEFT | Fuer folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wird ein Mistrade-Antrag geprueft:ISIN Datum Zeit Volumen Preis Fair Value (AS)DE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 3,986 | +0,61 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| AMGEN | 325,15 | +0,06 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| NOVAVAX | 6,910 | -0,80 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| STRYKER | 302,10 | -0,33 % | Stryker Corporation: Stryker declares an $0.88 per share quarterly dividend | Portage, Michigan, Feb. 04, 2026 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) announced that its Board of Directors has declared a quarterly dividend of $0.88 per share payable April 30, 2026, to shareholders... ► Artikel lesen | |
| BIOGEN | 164,25 | +3,79 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,420 | -1,22 % | PTA-news: Biofrontera AG: Biofrontera berichtet über die Ergebnisse der ersten neun Monate des Jahres 2025 | DJ PTA-News: Biofrontera AG: BIOFRONTERA BERICHTET ÜBER DIE ERGEBNISSE DER ERSTEN NEUN MONATE DES JAHRES 2025
Unternehmensmitteilung für den Kapitalmarkt
Biofrontera AG: BIOFRONTERA... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,910 | 0,00 % | HEIDELBERG PHARMA AG zeigt klare Stärke im Trend | ||
| ILLUMINA | 101,04 | -0,18 % | Illumina-Aktie -10%: Kann man hier günstig einsteigen? | Mit einem Kursverlust von -10% war die Illumina-Aktie am Freitag der große Verlierer im S&P 500. Der Hersteller von Gensequenzierungsmaschinen setzte damit seinen Abwärtstrend der letzten Tage fort.... ► Artikel lesen | |
| PRAXIS PRECISION MEDICINES | 319,57 | +4,56 % | Praxis Precision Medicines, Inc. Announces Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) |
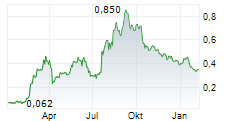
ANTENGENE CORP LTD gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von -0,004 (-1,15 %) liegt der Kurs bei 0,344 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ANTENGENE CORP LTD finden Sie auf Wallstreet Online.