- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mi | Aprea Therapeutics appoints Eugene Kennedy as chief medical advisor | 2 | Investing.com | ||
| APREA THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| Mi | Aprea Therapeutics Appoints Industry Veteran Eugene Kennedy, MD, as Chief Medical Advisor | 335 | GlobeNewswire (Europe) | DOYLESTOWN, Pa., Feb. 04, 2026 (GLOBE NEWSWIRE) -- Aprea Therapeutics, Inc. (Nasdaq: APRE) ("Aprea" or the "Company"), a clinical-stage biopharmaceutical company developing innovative therapies that... ► Artikel lesen | |
| 30.01. | Weekly Buzz: Intellia Gets FDA Nod For ATTRv-PN Trial; Aprea's APR-1051 Paces; CALC Halts KOURAGE | 585 | AFX News | SOUTH SAN FRANCISCO (dpa-AFX) - This week's biotech landscape features key FDA approvals, clinical holds and go-aheads, trial discontinuations, and clinical trial data readouts across key therapeutic... ► Artikel lesen | |
| 29.01. | Aprea meldet erstes partielles Ansprechen in Phase-1-Studie für Krebsmedikament | 7 | Investing.com Deutsch | ||
| 29.01. | Aprea Therapeutics prices $5.6M private placement | 7 | Seeking Alpha | ||
| 29.01. | Aprea Therapeutics raises $5.6 million in private placement | 6 | Investing.com | ||
| 29.01. | Aprea reports first partial response in Phase 1 cancer drug trial | 2 | Investing.com | ||
| 29.01. | Aprea Therapeutics Announces $5.6 Million Private Placement Priced At-The-Market Under Nasdaq Rules | 142 | GlobeNewswire (Europe) | DOYLESTOWN, Pa., Jan. 29, 2026 (GLOBE NEWSWIRE) -- Aprea Therapeutics, Inc. (Nasdaq: APRE) ("Aprea", or the "Company"), a clinical-stage biopharmaceutical company developing innovative treatments... ► Artikel lesen | |
| 29.01. | Aprea Therapeutics Announces Early Clinical Proof-Of-Concept in the Ongoing ACESOT-1051 Dose-Escalation Trial Evaluating WEE1 Inhibitor APR-1051, Including Partial Response Observed on First Scan | 122 | GlobeNewswire (Europe) | Approximately 50% reduction in target lesion and greater than 90% decrease in CA-125 observed in endometrial cancer patientThe unconfirmed partial response (uPR) that was observed in the first scan... ► Artikel lesen | |
| 23.01. | Aprea Therapeutics, Inc. - 8-K, Current Report | 4 | SEC Filings | ||
| 09.01. | Aprea Therapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 18.12.25 | Aprea Therapeutics reports progress in cancer drug development | 16 | Investing.com | ||
| 18.12.25 | Fortschritte bei Krebstherapie: Aprea Therapeutics meldet Studiendaten und sichert Liquidität | 11 | Investing.com Deutsch | ||
| 18.12.25 | Aprea Therapeutics CEO Issues Letter to Shareholders Highlighting Pipeline Progress in 2025 and Outlook for 2026 | 198 | GlobeNewswire (Europe) | DOYLESTOWN, Pa., Dec. 18, 2025 (GLOBE NEWSWIRE) -- Aprea Therapeutics, Inc. (Nasdaq: APRE) ("Aprea", or the "Company"), a clinical-stage biopharmaceutical company developing innovative treatments... ► Artikel lesen | |
| 09.12.25 | Aprea Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 09.12.25 | Aprea Therapeutics sichert sich 3,1 Mio. US-Dollar durch Privatplatzierung | 1 | Investing.com Deutsch | ||
| 09.12.25 | Aprea Therapeutics Announces $3.1 Million Private Placement Priced At-The-Market Under Nasdaq Rules | 3 | GlobeNewswire (USA) | ||
| 08.12.25 | Aprea Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | - | SEC Filings | ||
| 12.11.25 | Aprea Therapeutics, Inc. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 12.11.25 | Aprea Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings |
1 2 Weiter >>
34 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| EVOTEC | 6,148 | +0,79 % | SDAX: Evotec-Aktie schießt zweistellig nach oben | Eine Kaufempfehlung von Berenberg entfacht neuen Optimismus bei Evotec und katapultiert die Biotech-Aktie an die Indexspitze. Die Aktie der Evotec hat am Dienstagmittag ein Kursfeuerwerk gezündet und... ► Artikel lesen | |
| BB BIOTECH | 50,60 | -0,78 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| VALNEVA | 3,986 | +0,61 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| BIOGEN | 164,25 | +3,79 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| ILLUMINA | 101,04 | -0,18 % | Illumina-Aktie -10%: Kann man hier günstig einsteigen? | Mit einem Kursverlust von -10% war die Illumina-Aktie am Freitag der große Verlierer im S&P 500. Der Hersteller von Gensequenzierungsmaschinen setzte damit seinen Abwärtstrend der letzten Tage fort.... ► Artikel lesen | |
| ABIVAX | 98,30 | -0,30 % | ABIVAX Société Anonyme (ABVX): A Bull Case Theory | ||
| GINKGO BIOWORKS | 8,600 | +0,58 % | OpenAI und Ginkgo Bioworks optimieren Proteinsynthese mit GPT-5 im Roboterlabor | ||
| ONCO-INNOVATIONS | 0,535 | -0,93 % | Onco-Innovations meldet Einreichung eines vorläufigen Basisprospekts | Vancouver, Kanada - 4. Februar 2026 / IRW-Press / Onco-Innovations Limited (CBOE CA: ONCO) (OTCQB: ONNVF) (FWB: W1H, WKN: A3EKSZ) ("Onco" oder das "Unternehmen") gibt bekannt, dass es einen vorläufigen... ► Artikel lesen | |
| PRAXIS PRECISION MEDICINES | 319,57 | +4,56 % | Praxis Precision Medicines, Inc. Announces Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | ||
| QIAGEN | 43,170 | -0,70 % | Ionos, Kontron, Lanxess, Qiagen, Redcare Pharmacy, Siltronic, TeamViewer: Neue Aktien-Positionen der Short-Seller | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| AVIDITY BIOSCIENCES | 72,90 | +0,11 % | Avidity Biosciences, Inc. - 8-K, Current Report | ||
| TARSUS PHARMACEUTICALS | 64,62 | +4,24 % | Tarsus Pharmaceuticals, Inc: Tarsus Reports Third Quarter 2025 Financial Results and Recent Business Achievements | Delivered quarterly XDEMVY® net sales of approximately $119 million, up approximately 147% year-over-year Weekly multi-patient prescribers grew approximately 30% in the third quarter underscoring... ► Artikel lesen | |
| SUMMIT THERAPEUTICS | 14,990 | +8,23 % | H.C. Wainwright reiterates Buy rating on Summit Therapeutics stock | ||
| ERASCA | 12,290 | +3,45 % | Erasca, Inc.: Erasca Announces Pricing of Upsized Public Offering of Common Stock | SAN DIEGO, Jan. 21, 2026 (GLOBE NEWSWIRE) -- Erasca, Inc. (Nasdaq: ERAS), a clinical-stage precision oncology company singularly focused on discovering, developing, and commercializing therapies for... ► Artikel lesen | |
| AGOMAB THERAPEUTICS | 14,650 | 0,00 % | AgomAb Therapeutics N.V.: Agomab Announces Pricing of Initial Public Offering | ANTWERP, Belgium, February 5, 2026 (GLOBE NEWSWIRE) - Agomab Therapeutics NV (Nasdaq: AGMB) ("Agomab"), a clinical-stage biopharmaceutical company focused on developing novel disease-modifying therapies... ► Artikel lesen |
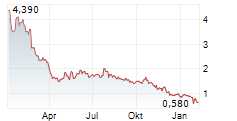
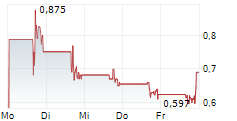
Das Unternehmen APREA THERAPEUTICS INC kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell +10,51 % bei einem Aktienkurs von 0,690 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu APREA THERAPEUTICS INC finden Sie auf Wallstreet Online.