- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | Weekly Buzz: ATYR To Meet With FDA, SGMO Aces Fabry Study, AQST's Anaphylm Gets Rejected | 346 | AFX News | PETAH TIKVA (dpa-AFX) - This week's biotech landscape witnessed key FDA meetings being scheduled, IND clearances, complete response letters, sale of oncology assets and clinical trial data readouts... ► Artikel lesen | |
| Di | H.C. Wainwright reiterates Buy rating on Aquestive stock after FDA response | 3 | Investing.com | ||
| Di | Aquestive receives FDA CRL for Anaphylm allergic reaction treatment | 2 | Pharmaceutical Technology | ||
| Mo | Aquestive jumps despite receiving FDA Complete Response Letter | 8 | Seeking Alpha | ||
| AQUESTIVE THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| Mo | What's Going On With Aquestive Therapeutics Stock After FDA Update For Lead Allergic Treatment? | 1 | Benzinga.com | ||
| Mo | With FDA rejections, Aquestive's shares go up, while Pharming's go down | 13 | FiercePharma | ||
| Mo | Aquestive Receives CRL For Anaphylm NDA; Deficiencies Limited To Packaging, Administration | 2 | RTTNews | ||
| Mo | Aquestive-Aktie steigt trotz vorläufiger FDA-Ablehnung für Anaphylaxie-Film | 6 | Investing.com Deutsch | ||
| Mo | FDA issues complete response letter for Aquestive's anaphylaxis drug | 1 | Investing.com | ||
| Mo | Aquestive Therapeutics, Inc.: Aquestive Therapeutics Announces FDA Issuance of Complete Response Letter for Anaphylm | 846 | GlobeNewswire (Europe) | Deficiencies limited to packaging and administrationCompany believes it can rapidly resolve deficiencies and expects to resubmit as early as Q3 2026Remains well-capitalized and anticipates ending... ► Artikel lesen | |
| Mo | Aquestive Therapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 21.01. | Aquestive Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | 6 | SEC Filings | ||
| 13.01. | Block & Leviton LLP: Aquestive Therapeutics Investors Who Have Lost Money Should Contact Block & Leviton to Find Out How They Might Recover Money Through the Firm's Investigation | 597 | Newsfile | Boston, Massachusetts--(Newsfile Corp. - January 13, 2026) - Block & Leviton is investigating Aquestive Therapeutics, Inc. (NASDAQ: AQST) for potential securities law violations. Investors who have... ► Artikel lesen | |
| 10.01. | Benzinga Bulls And Bears: Chevron, Palantir, Aquestive - And Real Estate Stocks Plummet | 29 | Benzinga.com | ||
| 09.01. | Aquestive Stock Slides After FDA Flags Issues With Allergy Drug Application | 12 | Benzinga.com | ||
| 09.01. | Aquestive drops as FDA flags issues in Anaphylm marketing application | 13 | Seeking Alpha | ||
| 09.01. | Aquestive: FDA stellt Mängel bei Zulassungsantrag für Anaphylm fest | 30 | Investing.com Deutsch | ||
| 09.01. | FDA identifies deficiencies in Aquestive's Anaphylm application | 4 | Investing.com | ||
| 09.01. | Aquestive Therapeutics, Inc.: Aquestive Therapeutics Announces Regulatory Development for Anaphylm (dibutepinephrine) Sublingual Film and Provides Business Update | 914 | GlobeNewswire (Europe) | Announces receipt of FDA letter stating it has identified deficiencies that preclude labeling discussions for Anaphylm at this time Receives confirmation from FDA that Agency's review of Anaphylm... ► Artikel lesen | |
| 09.01. | Aquestive Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings |
1 2 3 Weiter >>
57 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 45,800 | +2,20 % | Dax - Ausblick auf die neue Handelswoche: Bayer - Wann fällt die Marke von 50 Euro? | Nach einer überaus turbulenten Handelswoche richten sich die Blicke bereits auf die neue Handelswoche und damit auf den neuen Monat. Der Januar gestaltete sich für den Dax durchaus ambivalent. Dem Index... ► Artikel lesen | |
| NOVO NORDISK | 40,310 | +0,01 % | Abnehmpille schlägt ein: Starker Start ins neue Jahr - Novo Nordisk zeigt Eli Lilly die Rücklichter! | © Foto: Kristian Tuxen Ladegaard Berg - NurPhotoNach einem schwachen Vorjahr meldet sich Novo Nordisk 2026 eindrucksvoll zurück. Die neue Wegovy-Tablette sorgt in den USA für einen starken Marktstart.... ► Artikel lesen | |
| PFIZER | 23,015 | -0,07 % | Opening Bell: Gold/Silber, PepsiCo, Merck & Co, Pfizer, PayPal, Teradyne, Uber/Snap/Alphabet, Nvidia | Die US-Börsen sind zum Wochenstart fester aus dem Handel gegangen. Der Dow Jones gewann 1,05 Prozent, der Nasdaq Composite legte 0,56 Prozent zu. Am Freitag hatte die mögliche Nominierung von Kevin... ► Artikel lesen | |
| NOVARTIS | 132,06 | +0,03 % | Novartis macht über 17 Milliarden Gewinn | Basel - Novartis hat im Geschäftsjahr 2025 einen Gewinn von 17,4 Milliarden US-Dollar verbucht, wie der Pharmakonzern am Mittwoch mitteilte. Das entspricht einer Steigerung von 11 Prozent zum Vorjahr.... ► Artikel lesen | |
| MERCK KGAA | 121,45 | -0,49 % | BARCLAYS stuft MERCK KGAA auf 'Equal Weight' | LONDON (dpa-AFX Analyser) - Die britische Investmentbank Barclays hat die Einstufung für Merck KGaA mit einem Kursziel von 130 Euro auf "Equal Weight" belassen. Der Fokus liege auf möglichen Risiken... ► Artikel lesen | |
| GILEAD SCIENCES | 128,98 | -0,05 % | Gilead wins favorable U.S. label update for Yescarta | ||
| AURORA CANNABIS | 2,925 | -0,85 % | Aurora Cannabis Inc.: Aurora Cannabis Announces Fiscal 2026 Third Quarter Results | NASDAQ | TSX: ACB
Expands YoY Total Net Revenue1 by 7% to $94.2 million, with Global Medical Cannabis Net Revenue1 Increasing by 12% to a Record $76.2 million
... ► Artikel lesen | |
| SANOFI | 80,49 | +0,05 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| GSK | 25,300 | +1,12 % | GSK: Besser als erwartet | Der Pharmakonzern konnte ein unerwartet starkes Schlussquartal vorweisen und damit besser abschneiden als gedacht. Der Umsatz von GSK klettert im vergangenen Jahr um 4 % auf knapp 32,7 Mrd. Pfund, währungsbereinigt... ► Artikel lesen | |
| ABBVIE | 189,20 | +0,11 % | Aktien New York Ausblick: Keine Tech-Erholung - Eli Lilly steigen kräftig | NEW YORK (dpa-AFX) - Mit Blick auf den Technologiesektor dürften sich die Investoren an den US-Börsen am Mittwoch zurückhalten. Der Nasdaq 100 Index, der am Vortag deutlich gefallen war, dürfte sich... ► Artikel lesen | |
| CANOPY GROWTH | 0,935 | -0,95 % | Canopy Growth: Treffer ohne Gewinn - Trading-Tipp wird zurückgezogen | ||
| ROCHE | 391,10 | -0,29 % | DZ BANK stuft ROCHE HOLDINGS AG auf 'Kaufen' | FRANKFURT (dpa-AFX Analyser) - Die DZ Bank hat den fairen Aktienwert für Roche von 324 auf 395 Franken angehoben und die Einstufung auf "Kaufen" belassen. "Die Pharma-Sparte hat den großen, nahezu parallelen... ► Artikel lesen | |
| ELI LILLY | 894,80 | -0,01 % | Goldman Sachs Reaffirms Buy on Eli Lilly and Company (LLY), Citing 25% Growth Outlook | ||
| ASTRAZENECA | 160,70 | 0,00 % | Erfolg für AstraZeneca | Der Pharmakonzern hat gestern gemeldet, dass sein Medikament Imfinzi in Kombination mit einer Chemotherapie für die Behandlung erwachsener Patienten mit Magen- sowie gastroösophagealen Übergangstumoren... ► Artikel lesen | |
| TILRAY BRANDS | 6,450 | +0,62 % | Tilray Brands, Inc.: CC Pharma von Tilray als eines der 100 innovativsten Unternehmen in Deutschland ausgezeichnet | Die Auszeichnung würdigt CC Pharma als innovativen Marktführer und vertrauenswürdigen Partner auf dem europäischen PharmamarktDENSBORN, Deutschland, Feb. 04, 2026 (GLOBE NEWSWIRE) -- Tilray Pharma,... ► Artikel lesen |
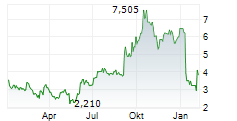
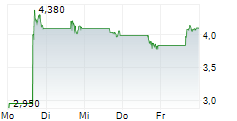
Das Unternehmen AQUESTIVE THERAPEUTICS INC kann der Branche Pharma zugeordnet werden. Die relative Kursveränderung beträgt aktuell +6,90 % bei einem Aktienkurs von 4,105 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu AQUESTIVE THERAPEUTICS INC finden Sie auf Wallstreet Online.