| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
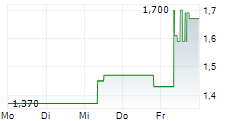
| 1,610 | 1,680 | 13:04 | |
| 1,590 | 1,690 | 06.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | Ascletis Pharma Inc.: GIC Invests US$100M in Ascletis Pharma (1672. HK): Anchoring Long-Term Capital in China's Core Innovative Drug Assets | 249 | JCN Newswire | HONG KONG, Feb 6, 2026 - (ACN Newswire) - According to disclosure of interests filed with the Hong Kong Stock Exchange (HKEX), the Government of Singapore Investment Corporation (GIC) has acquired an... ► Artikel lesen | |
| Fr | Ascletis Pharma Inc.: GIC Private Limited initiated a stake in Ascletis Pharma (01672) by 64,128,000 shares at a price of HKD 12.18 per share | 218 | JCN Newswire | HONG KONG, Feb 6, 2026 - (ACN Newswire) - According to the disclosure of interests information released by the Hong Kong Stock Exchange, on 5 February, GIC Private Limited made its first equity investment... ► Artikel lesen | |
| Do | Ascletis Pharma Inc.: GIC Private Limited initiated a stake in Ascletis Pharma (01672) by 64,128,000 shares at a price of HKD 12.18 per share | 112 | JCN Newswire | HONG KONG, Feb 5, 2026 - (ACN Newswire) - According to the disclosure of interests information released by the Hong Kong Stock Exchange, GIC Private Limited made its equity investment in Ascletis Pharma... ► Artikel lesen | |
| Do | Sagimet announces positive results for Ascletis' denifanstat in moderate to severe acne | 5 | PMLiVE | ||
| Di | ASCLETIS-B (01672): PLACING OF NEW SHARES UNDER THE GENERAL MANDATE | 2 | HKEx | ||
| Mo | Ascletis reports positive long-term safety data for acne drug denifanstat | 4 | Investing.com | ||
| ASCLETIS PHARMA Aktie jetzt für 0€ handeln | |||||
| Mo | Sagimet Biosciences Inc.: Sagimet Announces Positive 52-Week Data from License Partner Ascletis' Open-Label Phase 3 Clinical Trial Evaluating the Long-Term Safety of ASC40 (Denifanstat) Tablets in Patients with Moderate to Severe Acne | 209 | GlobeNewswire (Europe) | SAN MATEO, Calif., Feb. 02, 2026 (GLOBE NEWSWIRE) -- Sagimet Biosciences Inc. (Nasdaq: SGMT), a clinical-stage biopharmaceutical company developing novel therapeutics targeting dysfunctional metabolic... ► Artikel lesen | |
| 29.01. | Ascletis Pharma Inc.: Ascletis Announces Positive Topline Results from Its Phase III Open-Label Study of Denifanstat (ASC40), a First-in-Class, Once-Daily Oral FASN Inhibitor for Acne | 209 | PR Newswire | - Denifanstat (ASC40), a once-daily oral fatty acid synthase (FASN) inhibitor, demonstrated favorable safety and tolerability in a Phase III open-label study
-... ► Artikel lesen | |
| 29.01. | ASCLETIS-B (01672): VOLUNTARY ANNOUNCEMENT - ASCLETIS ANNOUNCES POSITIVE TOPLINE RESULTS FROM ITS PHASE III OPEN-LABEL STUDY OF DENIFANSTAT (ASC40), A ... | - | HKEx | ||
| 26.01. | Ascletis Pharma Inc.: Ascletis Announces First Participants Dosed in a 13-week U.S. Phase II Study with ASC30, an Oral Small Molecule GLP-1R Agonist for the Treatment of Diabetes | 105 | PR Newswire | - Topline data from the Phase II study for the treatment of diabetes are expected in the third quarter of 2026.
-ASC30 demonstrated placebo-adjusted weight loss... ► Artikel lesen | |
| 26.01. | ASCLETIS-B (01672): VOLUNTARY ANNOUNCEMENT - ASCLETIS ANNOUNCES FIRST PARTICIPANTS DOSED IN A 13-WEEK U.S. PHASE II STUDY WITH ASC30, AN ORAL SMALL MOLECULE ... | 3 | HKEx | ||
| 20.01. | ASCLETIS-B (01672): VOLUNTARY ANNOUNCEMENT - ASCLETIS SELECTS A NEXT-GENERATION ONCE-MONTHLY SUBCUTANEOUSLY ADMINISTERED GLP-1R/GIPR/GCGR TRIPLE PEPTIDE ... | - | HKEx | ||
| 20.01. | Ascletis Pharma Inc.: Ascletis Selects a Next-Generation Once-Monthly Subcutaneously Administered GLP-1R/GIPR/GCGR Triple Peptide Agonist, ASC37, for Clinical Development | 188 | PR Newswire | - In head-to-head non-human primate (NHP) studies, average observed half-life of ASC37 was approximately 17 days, 7-fold longer than retatrutide, which supports... ► Artikel lesen | |
| 05.01. | Ascletis Pharma Inc.: Ascletis Announces U.S. FDA IND Clearance for 13-Week Phase II Study of Its Oral Small Molecule GLP-1, ASC30, in Participants with Diabetes | 155 | PR Newswire | -The Phase II study for diabetes is a 13-week, randomized, double-blind, placebo-controlled and multi-center study to evaluate the efficacy, safety, and tolerability... ► Artikel lesen | |
| 05.01. | ASCLETIS-B (01672): VOLUNTARY ANNOUNCEMENT - ASCLETIS ANNOUNCES U.S. FDA IND CLEARANCE FOR 13-WEEK PHASE II STUDY OF ITS ORAL SMALL MOLECULE GLP-1, ASC30, ... | 1 | HKEx | ||
| 29.12.25 | ASCLETIS-B (01672): NEXT DAY DISCLOSURE RETURN | 1 | HKEx | ||
| 19.12.25 | ASCLETIS-B (01672): NEXT DAY DISCLOSURE RETURN | - | HKEx | ||
| 16.12.25 | ASCLETIS-B (01672): VOLUNTARY ANNOUNCEMENT - UPSIZING OF PROPOSED SHARE REPURCHASE UNDER THE REPURCHASE MANDATE | - | HKEx | ||
| 15.12.25 | Ascletis Pharma Inc.: Ascletis Announces Positive Topline Results from U.S. Phase I Study of ASC50, a Potential Best-in-Class Oral Small Molecule IL-17 Inhibitor | 325 | PR Newswire | - The elimination half-life of ASC50 after a single oral dose was 43, 89, 91, 87, 104, and 85 hours for 10 mg, 30 mg, 100 mg, 200 mg, 400 mg, and 600 mg, respectively... ► Artikel lesen | |
| 15.12.25 | ASCLETIS-B (01672): VOLUNTARY ANNOUNCEMENT - ASCLETIS ANNOUNCES POSITIVE TOPLINE RESULTS FROM U.S. PHASE I STUDY OF ASC50, A POTENTIAL BEST-IN-CLASS ORAL ... | 1 | HKEx |
1 2 3 4 5 Weiter >>
81 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 90,40 | +0,17 % | BioNTech- und Nvidia-Aktien verkauft, Medline und Alphabet rein: Erste 13F-Filings sind da! | Der britische Vermögensverwalter Baillie Gifford hat als einer der ersten sein 13F-Filing für das 4. Quartal abgegeben. Unter den großen Namen im Portfolio gibt es ein paar spannende Veränderungen.... ► Artikel lesen | |
| EVOTEC | 6,148 | +0,79 % | SDAX: Evotec-Aktie schießt zweistellig nach oben | Eine Kaufempfehlung von Berenberg entfacht neuen Optimismus bei Evotec und katapultiert die Biotech-Aktie an die Indexspitze. Die Aktie der Evotec hat am Dienstagmittag ein Kursfeuerwerk gezündet und... ► Artikel lesen | |
| BB BIOTECH | 50,60 | -0,78 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| MEDIGENE | 0,042 | -8,26 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 43,170 | -0,70 % | JEFFERIES stuft QIAGEN NV auf 'Buy' | NEW YORK (dpa-AFX Analyser) - Das Analysehaus Jefferies hat die Einstufung für Qiagen mit einem Kursziel von 57 US-Dollar auf "Buy" belassen. Umsatz und Ergebnis des vierten Quartals hätten die Erwartungen... ► Artikel lesen | |
| MODERNA | 34,650 | -0,07 % | Arbutus jumps on ruling in patent dispute with Moderna | ||
| PAION | 0,200 | +8,11 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WIRD GEPRUEFT | Fuer folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wird ein Mistrade-Antrag geprueft:ISIN Datum Zeit Volumen Preis Fair Value (AS)DE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 3,986 | +0,61 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| AMGEN | 325,15 | +0,06 % | Märkte am Morgen: "KI-Realitätscheck", Wolters Kluwer, AMD, Amgen, Walmart, Novo Nordisk | Die Stimmung an den Aktienmärkten ist derzeit sehr, sehr wechselhaft. Das war gestern deutlich zu spüren. Der DAX war bemerkenswert stark in den Handel gestartet, schloss allerdings leicht im Minus.... ► Artikel lesen | |
| EPIGENOMICS | 0,870 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 6,910 | -0,80 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| STRYKER | 302,10 | -0,33 % | Stryker Corporation: Stryker declares an $0.88 per share quarterly dividend | Portage, Michigan, Feb. 04, 2026 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) announced that its Board of Directors has declared a quarterly dividend of $0.88 per share payable April 30, 2026, to shareholders... ► Artikel lesen | |
| BIOGEN | 164,25 | +3,79 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,420 | -1,22 % | PTA-news: Biofrontera AG: Biofrontera berichtet über die Ergebnisse der ersten neun Monate des Jahres 2025 | DJ PTA-News: Biofrontera AG: BIOFRONTERA BERICHTET ÜBER DIE ERGEBNISSE DER ERSTEN NEUN MONATE DES JAHRES 2025
Unternehmensmitteilung für den Kapitalmarkt
Biofrontera AG: BIOFRONTERA... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,910 | 0,00 % | HEIDELBERG PHARMA AG zeigt klare Stärke im Trend |
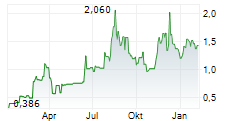
ASCLETIS PHARMA INC gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell +1,83 % bei einem Aktienkurs von 1,670 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ASCLETIS PHARMA INC finden Sie auf Wallstreet Online.