- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| ASPIRE BIOPHARMA Aktie jetzt für 0€ handeln | |||||
| Fr | Aspire Biopharma Holdings, Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| Mo | Aspire Biopharma Holdings, Inc., to Present at the Emerging Growth Conference on Thursday, April 17 | 169 | ACCESS Newswire | HUMACAO, PR AND NEW YORK, NY / ACCESS Newswire / April 14, 2025 / Aspire Biopharma Holdings, Inc. (Nasdaq:ASBP) ("Aspire" or the "Company"), a developer of a multi-faceted patent-pending drug delivery... ► Artikel lesen | |
| 11.04. | Aspire Biopharma beginnt mit der Produktion von Pre-Workout-Nahrungsergänzungsmitteln | - | Investing.com Deutsch | ||
| 11.04. | Aspire Biopharma starts production of pre-workout supplement | 2 | Investing.com | ||
| 11.04. | Aspire Biopharma Holdings, Inc., Commences Initial Production of its Pre-Workout Performance Supplement | 155 | ACCESS Newswire | Plans to conduct consumer and safety testing during second quarter 2025Desert Stream Inc.,a leading private label manufacturer in the health and wellness industry, performing initial productionSingle... ► Artikel lesen | |
| 09.04. | Aspire Biopharma Holdings, Inc., Announces Phase 1 Clinical Trial Initiation of its Oral Transmucosal Fast-Acting High-Dose Aspirin Formulation | 200 | ACCESS Newswire | One clinical trial site activatedPatient enrollment expected in AprilTrial designed to evaluate safety, pharmacokinetics and pharmacodynamics, of Aspire's sublingual aspirin productAspire anticipates... ► Artikel lesen | |
| 09.04. | Aspire Biopharma Holdings, Inc. - S-1, General form for registration of securities | 4 | SEC Filings | ||
| 07.04. | Aspire Biopharma Holdings, Inc. - 10-K, Annual Report | 1 | SEC Filings | ||
| 31.03. | Aspire Biopharma Holdings, Inc. - NT 10-K, Notification of inability to timely file Form 10-K 405, 10-K, 10-KSB 405, 10-KSB, 10-KT, or 10-KT405 | 1 | SEC Filings | ||
| 20.03. | Aspire Biopharma Holdings, Inc., Contract Manufacturer, Glatt Air Techniques, Inc., has Completed Its First Good Manufacturing Practice Clinical Batch of Pharmaceutical Grade Oral Mucosal Fast Acting Formulation of Aspirin | 205 | ACCESS Newswire | Subsequent phase involves completion of chemistry, manufacturing, and controls (CMC) testing for data to support FDA submissionCompany expects to commence bioavailability study in April 2025Aspire intends... ► Artikel lesen | |
| 13.03. | Aspire Biopharma treibt Patentanmeldungen für Arzneimittelverabreichungstechnologie voran | 5 | Investing.com Deutsch | ||
| 13.03. | Aspire Biopharma Holdings, Inc., Provides Update on Recently Filed U.S. Patent Applications for Its Sublingual Drug Delivery Platform | 216 | ACCESS Newswire | HUMACAO, PUERTO RICO AND NEW YORK, NY / ACCESS Newswire / March 13, 2025 / Aspire Biopharma Holdings, Inc. (NASDAQ:ASBP) ("Aspire" or the "Company"), a developer of a multi-faceted patent-pending drug... ► Artikel lesen | |
| 11.03. | SCD Media, LLC: An Interview with the CEO: How Aspire Biopharma is Disrupting a $100 Billion Market with Sublingual Innovation | 177 | Newsfile | New York, New York--(Newsfile Corp. - March 11, 2025) - Aspire Biopharma Holdings, Inc. (NASDAQ: ASBP) ("Aspire" or the "Company") is revolutionizing drug delivery through its proprietary sublingual... ► Artikel lesen | |
| 03.03. | Aspire Biopharma Holdings, Inc., to Launch the Next Generation of Pre-Workout Performance Supplement | 206 | ACCESS Newswire | New product expected to set a new standard for potency and performance in the estimated $20 billon pre-workout marketDesert Stream Inc., a well-established nutritional and supplement manufacturer of... ► Artikel lesen | |
| 25.02. | Aspire Biopharma advances to phase 1 clinical trial for aspirin product | 2 | Investing.com | ||
| 25.02. | Aspire Biopharma startet Phase-1-Studie für Aspirin-Produkt | 3 | Investing.com Deutsch | ||
| 25.02. | Aspire Biopharma, Inc.: Aspire Biopharma Holdings, Inc., Provides an Update on Lead Product Candidate: High-Dose, Sublingual Aspirin | 354 | ACCESS Newswire | Initial feasibility study of its soluble, Ph neutral, fast acting granular or powder drug delivery system is completeTest results support the next development step with Phase 1clinical trial expected... ► Artikel lesen | |
| 21.02. | Aspire Biopharma, Inc.: Aspire Biopharma Holdings, Inc. Announces the Execution of Securities Purchase Agreement | 523 | ACCESS Newswire | NEW YORK CITY, NY / ACCESS Newswire / February 20, 2025 / Aspire Biopharma, Inc. ("Aspire" or the "Company"), a developer of a multi-faceted patent-protected disruptive drug delivery mechanism technology... ► Artikel lesen | |
| 20.02. | Aspire Biopharma Holdings, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 20.02. | Aspire Biopharma announces public listing on Nasdaq | 2 | Seeking Alpha |
Seite:
1 2 Weiter >>
29 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| NOVO NORDISK | 51,88 | -8,68 % | Wachstum trotz Zölle, hier geht's rund: Künstliche Intelligenz nutzen! Novo Nordisk, Netramark Holdings, Infineon und Intel | Die Zollpolitik der US-Administration unter Trump wird die globale Wirtschaft negativ beeinflussen und birgt sogar die Gefahr einer neuen Weltwirtschaftskrise, so der ifo-Chef Clemens Fuest. Nur wenige... ► Artikel lesen | |
| GSK | 15,710 | -0,32 % | Kurz vor RSV-Impfdurchbruch: GSK: Goldman Sachs geht von einer positiven Überraschung in dieser Woche aus | © Foto: Nikos Pekiaridis - NurPhotoGoldman Sachs erwartet in dieser Woche eine positive Überraschung für den britischen Pharmakonzern GSK.Hintergrund ist eine bevorstehende Sitzung des Beratenden Ausschusses... ► Artikel lesen | |
| ROCHE | 273,85 | +0,74 % | JPMORGAN stuft ROCHE HOLDINGS AG auf 'Underweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für Roche mit einem Kursziel von 230 Franken auf "Underweight" belassen. Analyst Richard Vosser rechnet damit, dass der Umsatz der... ► Artikel lesen | |
| ELI LILLY | 739,30 | +12,41 % | Nachfrage nach Eli Lilly verhilft S&P 500 in die Gewinnzone (5.322 Pkt.) | Leichte Kursgewinne verzeichnen zur Stunde die Papiere im S&P 500-Index . Das Aktienbarometer steigt um 0,88 Prozent auf 5.322 Punkte. Die Bullen haben gegenwärtig an der Börse überwiegend das Sagen.... ► Artikel lesen | |
| TEVA | 12,150 | +1,25 % | Bausch Health Companies Inc.: U.S. District Court Grants Summary Judgment in Favor of FDA, Salix, and Teva, and Against Norwich | LAVAL, QC / ACCESS Newswire / April 17, 2025 / Bausch Health Companies Inc. (NYSE:BHC)(TSX:BHC) (the "Company" or "Bausch Health"), and its gastroenterology business Salix Pharmaceuticals, Inc., today... ► Artikel lesen | |
| VERTEX PHARMACEUTICALS | 432,00 | +0,48 % | Vertex Gains European Approval For Expanded Use Of KAFTRIO In Treating Cystic Fibrosis | CAMBRIDGE (MASSACHUSETTS) (dpa-AFX) - Vertex Pharmaceuticals (VRTX) announced that the European Commission has approved a label expansion for KAFTRIO (ivacaftor/tezacaftor/elexacaftor) in combination... ► Artikel lesen | |
| QUANTUM BIOPHARMA | 5,550 | +0,91 % | Quantum BioPharma Ltd.: Quantum BioPharma Corporate Update | Toronto, Ontario--(Newsfile Corp. - April 18, 2025) - Quantum BioPharma Ltd. (NASDAQ: QNTM) (CSE: QNTM) (FSE: 0K91) (Upstream: QNTM) ("Quantum BioPharma" or the "Company"), a biopharmaceutical company... ► Artikel lesen | |
| CSPC PHARMA | 0,602 | -2,21 % | Aktienmarkt: CSPC Pharmaceutical Group-Aktie kann sich nicht behaupten (0,6116 €) | Im Wertpapierhandel notiert das Wertpapier der CSPC Pharmaceutical Group derzeit leichter. Das Papier kostete zuletzt 0,61 Euro. Jahreschart der CSPC Pharmaceutical Group Ltd-Aktie, Stand 17.04.2025... ► Artikel lesen | |
| PENTIXAPHARM | 2,800 | 0,00 % | Pentixapharm verdoppelt Verluste: Zukunftsinvestition oder Geldverbrennung? | Das Berliner Biotech-Unternehmen Pentixapharm Holding AG (DE000A40AEG0) hat heute seine Jahreszahlen für 2024 veröffentlicht - und die künftigen Aussichten sorgen für Stirnrunzeln. Während der Konzern... ► Artikel lesen | |
| TONIX PHARMACEUTICALS | 14,000 | +1,45 % | Tonix Pharmaceuticals Holding Corp.: Tonix Pharmaceuticals Announces Oral Presentation and Panel Participation at the World Vaccine Congress Washington 2025 | ||
| ROCKET LAB USA | 17,372 | +0,06 % | Rocket Lab: Exorbitante Gewinne dank Verteidigungsboom | Unternehmen mit starker Anbindung an Verteidigungsorganisationen stehen bei Anlegern aktuell hoch im Kurs. Sie profitieren von wachsenden Budgets und steigenden Umsätzen - was sich auch an der Börse... ► Artikel lesen | |
| APONTIS PHARMA | 11,250 | +0,45 % | Aktien KW 14 News. Trump-Hammer crasht die Märkte. Weiter? Oder wenn die Kanonen… News. Aixtron. Steyr. Cancom. DHL. ProCredit. Energiekontor. Nordex. Biotest. Vossloh. Laiqon. Apontis. Drägerwerk. 2G Energy. All for One Group | Aktien Wochenrückblick - Trump hat diese Woche seinen "Boom" für die Aktienmärkte geschafft. Ob er an einen solchen Crash dachte, sei dahingestellt. Auf jeden Fall hat sein Zoll-Hammer die Märkte durchgeschüttelt.... ► Artikel lesen | |
| XORTX THERAPEUTICS | 0,790 | -2,95 % | XORTX Therapeutics Inc.: XORTX Announces Receipt of Nasdaq Notification Regarding Minimum Bid Price Deficiency | CALGARY, Alberta, April 17, 2025 (GLOBE NEWSWIRE) -- XORTX Therapeutics Inc. ("XORTX" or the "Company") (NASDAQ: XRTX | TSXV: XRTX | Frankfurt: ANU), a late stage clinical pharmaceutical company focused... ► Artikel lesen | |
| CATALYST PHARMACEUTICALS | 19,805 | -0,10 % | Catalyst Pharmaceuticals, Inc.: Catalyst Pharmaceuticals Announces Health Canada's Acceptance of AGAMREE New Drug Submission with Priority Review for Sub-Licensee Kye Pharmaceuticals | CORAL GABLES, Fla., April 08, 2025 (GLOBE NEWSWIRE) -- Catalyst Pharmaceuticals, Inc. ("Catalyst" or "Company") (Nasdaq: CPRX), a commercial-stage biopharmaceutical company focused on in-licensing... ► Artikel lesen | |
| TG THERAPEUTICS | 33,840 | +0,48 % | Price Over Earnings Overview: TG Therapeutics |
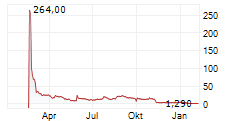
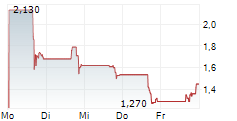
Das Unternehmen ASPIRE BIOPHARMA HOLDINGS INC kann der Branche Pharma zugeordnet werden. Die relative Kursveränderung beträgt aktuell -1,82 % bei einem Aktienkurs von 0,540 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und .
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ASPIRE BIOPHARMA HOLDINGS INC finden Sie auf Wallstreet Online.