- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Do | Astellas tops expectations as Vyloy sales surge outshines trial setback | 3 | FiercePharma | ||
| Mi | Earnings Call Transkript: Astellas Pharma meldet Umsatzplus für Q3 2025, Aktie steigt | 6 | Investing.com Deutsch | ||
| Mi | Astellas Pharma ADR GAAP EPS of ¥185.20, revenue of ¥1601.32B; updates full-year forecast | 16 | Seeking Alpha | ||
| 15.01. | JPM26: Astellas CEO resists 'rescue BD' as $6B Xtandi patent cliff nears | 17 | FiercePharma | ||
| 15.01. | Ocugen posts midphase gene therapy data, charting course for rivalry with Apellis, Astellas | 37 | FierceBiotech | ||
| ASTELLAS PHARMA INC ADR Aktie jetzt für 0€ handeln | |||||
| 08.01. | Pfizer, Astellas Report Positive Phase 3 Trial Results for PADCEV Combination in Bladder Cancer Treatment | 18 | Insider Monkey | ||
| 18.12.25 | Astellas Reports Positive Phase 3 EV-304 Results For PADCEV Combo In Muscle-Invasive Bladder Cancer | 487 | AFX News | NEW YORK CITY (dpa-AFX) - Astellas Pharma Inc. and Pfizer Inc. announced positive topline results from the Phase 3 EV-304 clinical trial (KEYNOTE-B15), evaluating PADCEV (enfortumab vedotin)... ► Artikel lesen | |
| 17.12.25 | Pfizer, Astellas post late-stage trial win for Padcev in bladder cancer with Merck's Keytruda | 10 | Seeking Alpha | ||
| 17.12.25 | Astellas Pharma Inc.: PADCEV Plus Keytruda Significantly Improves Survival for Patients with Muscle-Invasive Bladder Cancer Regardless of Cisplatin Eligibility | 332 | PR Newswire | PADCEV plus Keytruda is the first and only regimen without platinum-based chemotherapy to improve event-free and overall survival when used before and after surgery... ► Artikel lesen | |
| 15.12.25 | Aviceda geographic atrophy hopeful fails to top Astellas med in phase 2 | 5 | FierceBiotech | ||
| 01.12.25 | EMA Validates Review Of Astellas' PADCEV And KEYTRUDA Combination In Bladder Cancer | 762 | AFX News | TOKYO (dpa-AFX) - Astellas Pharma Inc. (ALPMY, 4503.T) announced that the European Medicines Agency (EMA) has validated for review a Type II variation application for PADCEV (enfortumab vedotin).... ► Artikel lesen | |
| 31.10.25 | Ajinomoto, Astellas Enter ADC Technology Agreement | 3 | Contract Pharma | ||
| 30.10.25 | Astellas Pharma gives H1 results | 18 | Seeking Alpha | ||
| 30.10.25 | Astellas Pharma H1 Net Profit Surges, Revenue Improves; Lifts Annual Outlook | 517 | AFX News | TOKYO (dpa-AFX) - Astellas Pharma Inc. (ALPMY, 4503.T), a Japanese pharmaceutical company, on Thursday reported a surge in net profit for the first half. In addition, the company has revised... ► Artikel lesen | |
| 27.10.25 | Bayer Menopause Drug Lands FDA Approval, Bringing Competition to an Astellas Product | 20 | MedCity News | ||
| 26.10.25 | Pfizer Inc. (PFE) and Astellas Pharma Announce Survival Results from Phase 3 EMBARK Study | 51 | Insider Monkey | ||
| 23.10.25 | Astellas taps social media star Babs Costello to promote Izervay for geographic atrophy | 6 | FiercePharma | ||
| 22.10.25 | Astellas Pharma Announces Encouraging Data From OPTION-VMS Phase IV Study | 2.816 | AFX News | TOKYO (dpa-AFX) - Astellas Pharma Inc. (ALPMY.PK), Wednesday announced new real-world preliminary data from the OPTION-VMS Phase IV study, a longitudinal, observational study in participants... ► Artikel lesen | |
| 22.10.25 | Astellas Pharma Inc.: Astellas Presents Preliminary Real-World VEOZAH (fezolinetant) Data From OPTION-VMS Phase IV Observational Study | 419 | PR Newswire | Extensive and growing evidence continues to reinforce fezolinetant as an effective treatment for moderate to severe VMS associated with menopause
Preliminary... ► Artikel lesen | |
| 22.10.25 | Astellas Pharma Inc.: PADCEV (enfortumab vedotin-ejfv) Plus KEYTRUDA (pembrolizumab) sBLA Granted FDA Priority Review for Treatment of Certain Patients with Muscle-Invasive Bladder Cancer | 353 | PR Newswire | Results from the pivotal EV-303 trial demonstrated that, when used before and after surgery, the combination reduced the risk of recurrence, progression or death... ► Artikel lesen |
1 2 3 Weiter >>
49 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 45,800 | +2,20 % | Lösung für Milliarden-Dollar-Problem: MustGrow Biologics validiert Geschäftsmodell - revolutionäre Meldung auch für Bayer und Corteva Agriscience | Raps ist für Kanada fast das, was Erdöl für Saudi-Arabien ist: Ein wirtschaftlicher Motor von gigantischem Ausmaß. Mit einem geschätzten Produktionswert von rund 14 Mrd. CAD im Jahr 2025 ist die gelb... ► Artikel lesen | |
| NOVO NORDISK | 40,310 | +0,01 % | Novo Nordisk Aktie: Aufgepasst, denn es wird spannend! Das Golden Cross rückt näher! | © Foto: 2026 Novo Nordisk A/SDie Novo Nordisk-Aktie steht vor einem entscheidenden charttechnischen Signal. Nach dem brutalen Absturz könnte sich das Blatt wenden. Der Kurs kämpft sich zurück, die wichtigen... ► Artikel lesen | |
| PFIZER | 23,015 | -0,07 % | Pfizer: Gute Zahlen, neue Daten - Kampfansage an Novo Nordisk und Co | Der Pharma-Gigant Pfizer hat am Dienstag die Zahlen für das abgelaufene vierte Quartal respektive Gesamtjahr 2025 veröffentlicht. Diese liegen über dem Markterwartungen. Darüber hinaus veröffentlichten... ► Artikel lesen | |
| NOVARTIS | 132,06 | +0,03 % | Novartis Pharma AG: Novartis erzielte im Jahr 2025 eine Umsatzsteigerung im hohen einstelligen Prozentbereich, eine Kerngewinnmarge von 40% sowie weitere Fortschritte in der Pipeline | Ad-hoc-Mitteilung gemäss Art. 53 KR
Geschäftsjahr
Der Nettoumsatz wuchs um +8% (kWk1, +8% USD), das operative Kernergebnis1 verbesserte sich um +14% (kWk, +12% USD)
Das Umsatzwachstum... ► Artikel lesen | |
| MERCK KGAA | 121,45 | -0,49 % | Merck KGaA: Aktie wird abgestuft | Am 5. März stehen bei Merck die Zahlen zum vierten Quartal an. Dann dürfte es auch eine Prognose für 2026 geben. Die Analysten der Deutschen Bank glauben, dass die Quartalszahlen keine Überraschungen... ► Artikel lesen | |
| GILEAD SCIENCES | 128,98 | -0,05 % | Gilead wins favorable U.S. label update for Yescarta | ||
| SANOFI | 80,49 | +0,05 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| GSK | 25,300 | +1,12 % | GSK: Besser als erwartet | Der Pharmakonzern konnte ein unerwartet starkes Schlussquartal vorweisen und damit besser abschneiden als gedacht. Der Umsatz von GSK klettert im vergangenen Jahr um 4 % auf knapp 32,7 Mrd. Pfund, währungsbereinigt... ► Artikel lesen | |
| ABBVIE | 189,20 | +0,11 % | Aktien New York Ausblick: Keine Tech-Erholung - Eli Lilly steigen kräftig | NEW YORK (dpa-AFX) - Mit Blick auf den Technologiesektor dürften sich die Investoren an den US-Börsen am Mittwoch zurückhalten. Der Nasdaq 100 Index, der am Vortag deutlich gefallen war, dürfte sich... ► Artikel lesen | |
| ROCHE | 391,10 | -0,29 % | DZ BANK stuft ROCHE HOLDINGS AG auf 'Kaufen' | FRANKFURT (dpa-AFX Analyser) - Die DZ Bank hat den fairen Aktienwert für Roche von 324 auf 395 Franken angehoben und die Einstufung auf "Kaufen" belassen. "Die Pharma-Sparte hat den großen, nahezu parallelen... ► Artikel lesen | |
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 894,80 | -0,01 % | Novo Nordisk-Aktie stürzt ab! Was macht Eli Lilly? Hochspannung vor Quartalszahlen des US-Konzerns | Der dänische Pharmariese Novo Nordisk steht unter massivem Druck: Umsatzwarnung, Kurssturz und scharfer internationaler Wettbewerb setzen dem Konzern zu. Anleger blicken nun gespannt auf die Zahlen... ► Artikel lesen | |
| MERCK & CO | 103,20 | 0,00 % | Merck & Co: Ausblick enttäuscht - Aktie unter Druck | Der US-amerikanische Pharma-Gigant Merck & Co hat am Dienstag die Zahlen für das abgelaufene vierte Quartal respektive Gesamtjahr 2025 veröffentlicht. Diese sind über den Erwartungen ausgefallen. Allerdings... ► Artikel lesen | |
| ASTRAZENECA | 160,70 | 0,00 % | Erfolg für AstraZeneca | Der Pharmakonzern hat gestern gemeldet, dass sein Medikament Imfinzi in Kombination mit einer Chemotherapie für die Behandlung erwachsener Patienten mit Magen- sowie gastroösophagealen Übergangstumoren... ► Artikel lesen | |
| BRISTOL-MYERS SQUIBB | 52,14 | -0,50 % | Aktien New York Ausblick: Für Dow sind 50.000 Punkte weiterhin zu hohe Hürde | NEW YORK (dpa-AFX) - Der Sprung über die runde Marke von 50.000 Punkten dürfte dem Dow Jones Industrial auch am Donnerstag zunächst versagt bleiben. Schwache Daten vom Arbeitsmarkt im Januar haben... ► Artikel lesen |
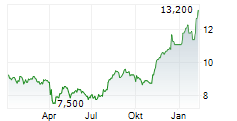
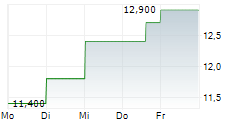
ASTELLAS PHARMA INC ADR gehört der Branche Pharma an. Die relative Kursveränderung beträgt aktuell +3,15 % bei einem Aktienkurs von 13,100 Euro. Die Dividendendrendite beträgt aktuell 0,00 % (berechnet anhand der Ausschüttung von 0,00 Euro in den letzten 12 Monaten).
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ASTELLAS PHARMA INC ADR finden Sie auf Wallstreet Online.