| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 13,400 | 13,615 | 13:05 | |
| 13,415 | 13,680 | 06.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Do | Astellas tops expectations as Vyloy sales surge outshines trial setback | 3 | FiercePharma | ||
| Mi | Earnings Call Transkript: Astellas Pharma meldet Umsatzplus für Q3 2025, Aktie steigt | 6 | Investing.com Deutsch | ||
| Mi | Astellas Pharma ADR GAAP EPS of ¥185.20, revenue of ¥1601.32B; updates full-year forecast | 16 | Seeking Alpha | ||
| 15.01. | JPM26: Astellas CEO resists 'rescue BD' as $6B Xtandi patent cliff nears | 17 | FiercePharma | ||
| 15.01. | Ocugen posts midphase gene therapy data, charting course for rivalry with Apellis, Astellas | 37 | FierceBiotech | ||
| 08.01. | Pfizer, Astellas Report Positive Phase 3 Trial Results for PADCEV Combination in Bladder Cancer Treatment | 18 | Insider Monkey | ||
| 18.12.25 | Astellas Reports Positive Phase 3 EV-304 Results For PADCEV Combo In Muscle-Invasive Bladder Cancer | 488 | AFX News | NEW YORK CITY (dpa-AFX) - Astellas Pharma Inc. and Pfizer Inc. announced positive topline results from the Phase 3 EV-304 clinical trial (KEYNOTE-B15), evaluating PADCEV (enfortumab vedotin)... ► Artikel lesen | |
| ASTELLAS PHARMA Aktie jetzt für 0€ handeln | |||||
| 17.12.25 | Pfizer, Astellas post late-stage trial win for Padcev in bladder cancer with Merck's Keytruda | 10 | Seeking Alpha | ||
| 17.12.25 | Astellas Pharma Inc.: PADCEV Plus Keytruda Significantly Improves Survival for Patients with Muscle-Invasive Bladder Cancer Regardless of Cisplatin Eligibility | 332 | PR Newswire | PADCEV plus Keytruda is the first and only regimen without platinum-based chemotherapy to improve event-free and overall survival when used before and after surgery... ► Artikel lesen | |
| 15.12.25 | Aviceda geographic atrophy hopeful fails to top Astellas med in phase 2 | 5 | FierceBiotech | ||
| 01.12.25 | EMA Validates Review Of Astellas' PADCEV And KEYTRUDA Combination In Bladder Cancer | 762 | AFX News | TOKYO (dpa-AFX) - Astellas Pharma Inc. (ALPMY, 4503.T) announced that the European Medicines Agency (EMA) has validated for review a Type II variation application for PADCEV (enfortumab vedotin).... ► Artikel lesen | |
| 31.10.25 | Ajinomoto, Astellas Enter ADC Technology Agreement | 3 | Contract Pharma | ||
| 30.10.25 | Astellas Pharma gives H1 results | 18 | Seeking Alpha | ||
| 30.10.25 | Astellas Pharma H1 Net Profit Surges, Revenue Improves; Lifts Annual Outlook | 517 | AFX News | TOKYO (dpa-AFX) - Astellas Pharma Inc. (ALPMY, 4503.T), a Japanese pharmaceutical company, on Thursday reported a surge in net profit for the first half. In addition, the company has revised... ► Artikel lesen | |
| 27.10.25 | Bayer Menopause Drug Lands FDA Approval, Bringing Competition to an Astellas Product | 20 | MedCity News | ||
| 26.10.25 | Pfizer Inc. (PFE) and Astellas Pharma Announce Survival Results from Phase 3 EMBARK Study | 51 | Insider Monkey | ||
| 23.10.25 | Astellas taps social media star Babs Costello to promote Izervay for geographic atrophy | 6 | FiercePharma | ||
| 22.10.25 | Astellas Pharma Announces Encouraging Data From OPTION-VMS Phase IV Study | 2.816 | AFX News | TOKYO (dpa-AFX) - Astellas Pharma Inc. (ALPMY.PK), Wednesday announced new real-world preliminary data from the OPTION-VMS Phase IV study, a longitudinal, observational study in participants... ► Artikel lesen | |
| 22.10.25 | Astellas Pharma Inc.: Astellas Presents Preliminary Real-World VEOZAH (fezolinetant) Data From OPTION-VMS Phase IV Observational Study | 419 | PR Newswire | Extensive and growing evidence continues to reinforce fezolinetant as an effective treatment for moderate to severe VMS associated with menopause
Preliminary... ► Artikel lesen | |
| 22.10.25 | Astellas Pharma Inc.: PADCEV (enfortumab vedotin-ejfv) Plus KEYTRUDA (pembrolizumab) sBLA Granted FDA Priority Review for Treatment of Certain Patients with Muscle-Invasive Bladder Cancer | 353 | PR Newswire | Results from the pivotal EV-303 trial demonstrated that, when used before and after surgery, the combination reduced the risk of recurrence, progression or death... ► Artikel lesen |
1 2 3 Weiter >>
51 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 45,800 | +2,20 % | Dax - Ausblick auf die neue Handelswoche: Bayer - Wann fällt die Marke von 50 Euro? | Nach einer überaus turbulenten Handelswoche richten sich die Blicke bereits auf die neue Handelswoche und damit auf den neuen Monat. Der Januar gestaltete sich für den Dax durchaus ambivalent. Dem Index... ► Artikel lesen | |
| NOVO NORDISK | 40,310 | +0,01 % | Abnehmpille schlägt ein: Starker Start ins neue Jahr - Novo Nordisk zeigt Eli Lilly die Rücklichter! | © Foto: Kristian Tuxen Ladegaard Berg - NurPhotoNach einem schwachen Vorjahr meldet sich Novo Nordisk 2026 eindrucksvoll zurück. Die neue Wegovy-Tablette sorgt in den USA für einen starken Marktstart.... ► Artikel lesen | |
| PFIZER | 23,015 | -0,07 % | Opening Bell: Gold/Silber, PepsiCo, Merck & Co, Pfizer, PayPal, Teradyne, Uber/Snap/Alphabet, Nvidia | Die US-Börsen sind zum Wochenstart fester aus dem Handel gegangen. Der Dow Jones gewann 1,05 Prozent, der Nasdaq Composite legte 0,56 Prozent zu. Am Freitag hatte die mögliche Nominierung von Kevin... ► Artikel lesen | |
| NOVARTIS | 132,06 | +0,03 % | Novartis macht über 17 Milliarden Gewinn | Basel - Novartis hat im Geschäftsjahr 2025 einen Gewinn von 17,4 Milliarden US-Dollar verbucht, wie der Pharmakonzern am Mittwoch mitteilte. Das entspricht einer Steigerung von 11 Prozent zum Vorjahr.... ► Artikel lesen | |
| MERCK KGAA | 121,45 | -0,49 % | BARCLAYS stuft MERCK KGAA auf 'Equal Weight' | LONDON (dpa-AFX Analyser) - Die britische Investmentbank Barclays hat die Einstufung für Merck KGaA mit einem Kursziel von 130 Euro auf "Equal Weight" belassen. Der Fokus liege auf möglichen Risiken... ► Artikel lesen | |
| GILEAD SCIENCES | 128,98 | -0,05 % | Gilead wins favorable U.S. label update for Yescarta | ||
| AURORA CANNABIS | 2,925 | -0,85 % | Aurora Cannabis Inc.: Aurora Cannabis Announces Fiscal 2026 Third Quarter Results | NASDAQ | TSX: ACB
Expands YoY Total Net Revenue1 by 7% to $94.2 million, with Global Medical Cannabis Net Revenue1 Increasing by 12% to a Record $76.2 million
... ► Artikel lesen | |
| SANOFI | 80,49 | +0,05 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| GSK | 25,300 | +1,12 % | GSK: Besser als erwartet | Der Pharmakonzern konnte ein unerwartet starkes Schlussquartal vorweisen und damit besser abschneiden als gedacht. Der Umsatz von GSK klettert im vergangenen Jahr um 4 % auf knapp 32,7 Mrd. Pfund, währungsbereinigt... ► Artikel lesen | |
| ABBVIE | 189,20 | +0,11 % | Aktien New York Ausblick: Keine Tech-Erholung - Eli Lilly steigen kräftig | NEW YORK (dpa-AFX) - Mit Blick auf den Technologiesektor dürften sich die Investoren an den US-Börsen am Mittwoch zurückhalten. Der Nasdaq 100 Index, der am Vortag deutlich gefallen war, dürfte sich... ► Artikel lesen | |
| CANOPY GROWTH | 0,935 | -0,95 % | Canopy Growth: Treffer ohne Gewinn - Trading-Tipp wird zurückgezogen | ||
| ROCHE | 391,10 | -0,29 % | DZ BANK stuft ROCHE HOLDINGS AG auf 'Kaufen' | FRANKFURT (dpa-AFX Analyser) - Die DZ Bank hat den fairen Aktienwert für Roche von 324 auf 395 Franken angehoben und die Einstufung auf "Kaufen" belassen. "Die Pharma-Sparte hat den großen, nahezu parallelen... ► Artikel lesen | |
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 894,80 | -0,01 % | Goldman Sachs Reaffirms Buy on Eli Lilly and Company (LLY), Citing 25% Growth Outlook | ||
| MERCK & CO | 103,20 | 0,00 % | Merck & Co. continues its bullish trend for the seventh straight session |
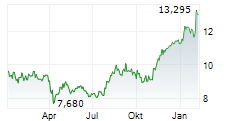
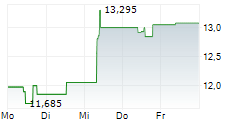
Das Unternehmen ASTELLAS PHARMA INC kann der Branche Pharma zugeordnet werden. Die relative Kursveränderung beträgt aktuell -3,58 % bei einem Aktienkurs von 13,070 Euro. Aus der Ausschüttung der letzten 12 Monate von insgesamt 0,41 Euro errechnet sich eine Dividendendrendite von +3,14 %.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ASTELLAS PHARMA INC finden Sie auf Wallstreet Online.