- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 11.04. | Biodexa Pharmaceuticals Plc - 20-F, Annual and transition report of foreign private issuers | 1 | SEC Filings | ||
| 11.04. | Biodexa Pharmaceuticals Plc - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 11.04. | Biodexa Pharmaceuticals PLC: Preliminary Results for the Year Ended 31 December 2024 | 128 | GlobeNewswire (Europe) | April 11, 2025 Biodexa Pharmaceuticals PLC("Biodexa" or the "Company" or, together with its subsidiaries, the "Group") Preliminary Results for the Year Ended 31 December 2024 Biodexa Pharmaceuticals... ► Artikel lesen | |
| 19.03. | Biodexa Pharmaceuticals PLC: With Fast Track Designation in Hand, a Successful Protocol Discussion with FDA, and CROs in Place, Biodexa is on Track to Initiate its Funded Phase 3 Trial in FAP Next Quarter | 299 | ACCESS Newswire | CARDIFF, UNITED KINGDOM / ACCESS Newswire / March 19, 2025 / Biodexa Pharmaceuticals PLC. (NASDAQ:BDRX), a clinical stage biopharmaceutical company developing a pipeline of innovative products for the... ► Artikel lesen | |
| 10.03. | Biodexa erhält FDA-Zustimmung für Phase-3-Studie von eRapa bei FAP | 4 | Investing.com Deutsch | ||
| 10.03. | Biodexa Pharmaceuticals PLC: Biodexa Announces Successful Outcome of a Type C Meeting with FDA Regarding the Phase 3 Program for eRapa in FAP | 345 | GlobeNewswire (Europe) | March 10, 2025 Biodexa Announces Successful Outcome of a Type C Meeting with FDA Regarding the Phase 3 Program for eRapa in FAP Clears the way to finalize Phase 3 protocol and recruit sites for U.S.... ► Artikel lesen | |
| 06.03. | Biodexa selects Precision as CRO for eRapa Phase 3 study in Europe | 1 | Investing.com | ||
| BIODEXA PHARMACEUTICALS Aktie jetzt für 0€ handeln | |||||
| 06.03. | Biodexa Pharmaceuticals PLC: Biodexa Announces Appointment of Precision for Medicine LLC as CRO for European Component of Phase 3 Study of eRapa in FAP | 145 | GlobeNewswire (Europe) | March 6, 2025 Biodexa Announces Appointment of Precision for Medicine LLC as CRO for European Component of Phase 3 Study of eRapa in FAP Biodexa is finalizing plans to initiate an international Phase... ► Artikel lesen | |
| 06.03. | Biodexa Pharmaceuticals Plc - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 24.02. | Biodexa Pharmaceuticals PLC: Biodexa Announces Allowance of U.S. Patent Covering Oral Rapamycin Nanoparticle Preparations ("eRapa") and Use | 160 | GlobeNewswire (Europe) | February 24, 2025 Biodexa Announces Allowance of U.S. Patent Covering Oral Rapamycin Nanoparticle Preparations ("eRapa") and Use Biodexa plans to initiate a Phase 3 registrational study of eRapa in... ► Artikel lesen | |
| 24.02. | Biodexa Pharmaceuticals Plc - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 12.02. | Biodexa Pharmaceuticals PLC: Following Positive Phase 2 Results and Orphan Drug Designation, Biodexa's FAP Drug Receives FDA Fast Track Status | 305 | ACCESS Newswire | CARDIFF, UK / ACCESS Newswire / February 12, 2025 / Biodexa Pharmaceuticals PLC. (NASDAQ:BDRX), a clinical-stage biopharmaceutical company developing a pipeline of innovative products for the treatment... ► Artikel lesen | |
| 10.02. | Biodexa stock soars 76% on FDA Fast Track status for eRapa | 10 | Seeking Alpha | ||
| 10.02. | Biodexa Pharmaceuticals PLC: Biodexa Receives US FDA Fast Track Designation for eRapa in Familial Adenomatous Polyposis | 172 | GlobeNewswire (Europe) | February 10, 2025 Biodexa Receives US FDA Fast Track Designation for eRapa in Familial Adenomatous Polyposis Underscores unmet need for a therapeutic alternative with the potential to delay or prevent... ► Artikel lesen | |
| 10.02. | Biodexa Pharmaceuticals Plc - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 22.01. | Biodexa Pharmaceuticals Names Gary Shangold Chief Medical Officer | - | RTTNews | ||
| 22.01. | Biodexa appoints Gary A. Shangold as Chief Medical Officer | 2 | Seeking Alpha | ||
| 22.01. | Biodexa Pharmaceuticals PLC: Biodexa Strengthens Management Team - Appointment of Dr Gary A. Shangold as Chief Medical Officer | 168 | GlobeNewswire (Europe) | January 22, 2025 Biodexa Strengthens Management Team Appointment of Dr Gary A. Shangold as Chief Medical Officer Biodexa Pharmaceuticals PLC ("Biodexa" or "the Company"), (Nasdaq: BDRX), a clinical... ► Artikel lesen | |
| 17.01. | Biodexa Pharmaceuticals files to sell 35.9M ADS for holders | 2 | Seeking Alpha | ||
| 17.01. | Biodexa Pharmaceuticals Plc - F-1, Registration statement for certain foreign private issuers | - | SEC Filings |
Seite:
1 2 3 Weiter >>
49 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 21,030 | +0,36 % | EQS-PVR: Bayer Aktiengesellschaft: Veröffentlichung gemäß § 40 Abs. 1 WpHG mit dem Ziel der europaweiten Verbreitung | EQS Stimmrechtsmitteilung: Bayer Aktiengesellschaft
Bayer Aktiengesellschaft: Veröffentlichung gemäß § 40 Abs. 1 WpHG mit dem Ziel der europaweiten Verbreitung
14.04.2025... ► Artikel lesen | |
| NOVO NORDISK | 51,88 | -8,68 % | Wachstum trotz Zölle, hier geht's rund: Künstliche Intelligenz nutzen! Novo Nordisk, Netramark Holdings, Infineon und Intel | Die Zollpolitik der US-Administration unter Trump wird die globale Wirtschaft negativ beeinflussen und birgt sogar die Gefahr einer neuen Weltwirtschaftskrise, so der ifo-Chef Clemens Fuest. Nur wenige... ► Artikel lesen | |
| PFIZER | 19,478 | +0,02 % | Pfizer stoppt Entwicklung von Abnehmpille | New York - Der Pharmakonzern Pfizer hat im heiss umkämpften Markt um Abnehm-Medikamente einen Rückschlag erlitten. Die Entwicklung einer von Investoren aufmerksam beobachteten Adipositas-Tablette wird... ► Artikel lesen | |
| NOVARTIS | 96,90 | -100,00 % | Novartis Aktie: Mega-Investition in US-Standorte | Der Schweizer Pharmakonzern plant eine Milliardeninvestition in amerikanische Standorte und schafft tausende Arbeitsplätze - Analysten bleiben dennoch zurückhaltend. Die Novartis-Aktie legte am Freitagvormittag... ► Artikel lesen | |
| MERCK KGAA | 116,65 | -1,69 % | Aktienmarkt: Kurs der Aktie von Merck im Plus (69,1413 €) | Im Plus liegt aktuell die Merck-Aktie . Zuletzt zahlten Investoren für das Papier 78,55 US-Dollar. Ein Preisanstieg von 2,73 Prozent steht gegenwärtig für das Wertpapier von Merck zu Buche. Die Aktie... ► Artikel lesen | |
| GILEAD SCIENCES | 91,99 | +0,08 % | Gilead Sciences: Advancing Oncology Care Through Gilead's Research Scholars Program | NORTHAMPTON, MA / ACCESS Newswire / April 15, 2025 / As a medical oncologist who has practiced in three different countries, Dr. Maria Alice Franzoi is well acquainted with various healthcare systems... ► Artikel lesen | |
| AURORA CANNABIS | 3,860 | -1,28 % | Aurora Cannabis Inc (3): Aurora Cannabis offers inhalers to U.K. patients | ||
| SANOFI | 90,51 | +0,11 % | Sanofi SA-Aktie: Kurs legt zu (91,41 €) | Die Aktie von Sanofi SA notiert heute etwas fester. Das Wertpapier notiert zur Stunde bei 91,41 Euro. Ein Kursplus von 1,97 Prozent steht gegenwärtig für die Sanofi SA-Aktie zu Buche. Das Papier verteuerte... ► Artikel lesen | |
| GSK | 15,710 | -0,32 % | Kurz vor RSV-Impfdurchbruch: GSK: Goldman Sachs geht von einer positiven Überraschung in dieser Woche aus | © Foto: Nikos Pekiaridis - NurPhotoGoldman Sachs erwartet in dieser Woche eine positive Überraschung für den britischen Pharmakonzern GSK.Hintergrund ist eine bevorstehende Sitzung des Beratenden Ausschusses... ► Artikel lesen | |
| ROCHE | 273,85 | +0,74 % | JPMORGAN stuft ROCHE HOLDINGS AG auf 'Underweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für Roche mit einem Kursziel von 230 Franken auf "Underweight" belassen. Analyst Richard Vosser rechnet damit, dass der Umsatz der... ► Artikel lesen | |
| CANOPY GROWTH | 1,052 | +0,77 % | Canopy Growth Aktie: Platzt am Montag die Bombe? Diese Neuigkeiten müssen Anleger dringend beachten! | ||
| STADA ARZNEIMITTEL | - | - | MIDDAY BRIEFING - Unternehmen und Märkte: CONTINENTAL, DAIMLER TRUCK, PORSCHE, HUGO BOSS, AUMANN, BAYWA, STADA, BYD, OMV, STELLANTIS | DJ MIDDAY BRIEFING - Unternehmen und Märkte
Der Markt-Überblick am Mittag, zusammengestellt von Dow Jones Newswires:
+++++ AKTIENMÄRKTE (13:18) +++++
zuletzt +/-... ► Artikel lesen | |
| ELI LILLY | 739,30 | +12,41 % | Nachfrage nach Eli Lilly verhilft S&P 500 in die Gewinnzone (5.322 Pkt.) | Leichte Kursgewinne verzeichnen zur Stunde die Papiere im S&P 500-Index . Das Aktienbarometer steigt um 0,88 Prozent auf 5.322 Punkte. Die Bullen haben gegenwärtig an der Börse überwiegend das Sagen.... ► Artikel lesen | |
| MERCK & CO | 68,60 | 0,00 % | Streak Analysis Alert: Why Merck (MRK) Should Be on Your Radar This Week | ||
| ASTRAZENECA | 118,75 | -0,92 % | Astrazeneca Aktie: Details zum strategischen Plan | Trotz aktueller Schwächephase halten Analysten an Kaufempfehlungen fest - Dividendenankündigung und Quartalszahlen im Fokus. Die AstraZeneca-Aktie zeigt sich diese Woche widersprüchlich: Während Aktionäre... ► Artikel lesen |
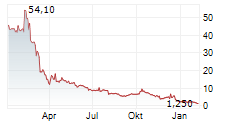
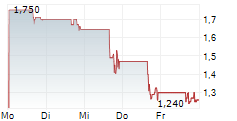
BIODEXA PHARMACEUTICALS PLC ADR gehört der Branche Pharma an. Die relative Kursveränderung beträgt aktuell -2,10 % bei einem Aktienkurs von 1,400 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und .
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu BIODEXA PHARMACEUTICALS PLC ADR finden Sie auf Wallstreet Online.