| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
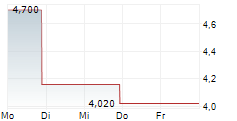
| 4,420 | 4,580 | 06.02. | |
| 4,400 | 4,620 | 06.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 08.01. | CervoMed Inc. - 8-K, Current Report | - | SEC Filings | ||
| 18.12.25 | Cantor Fitzgerald nimmt Aktie von CervoMed mit "Overweight" in die Bewertung auf | 3 | Investing.com Deutsch | ||
| 18.12.25 | Cantor Fitzgerald initiates CervoMed stock with Overweight rating | 2 | Investing.com | ||
| 05.12.25 | CervoMed Inc. - 8-K, Current Report | - | SEC Filings | ||
| 04.12.25 | CervoMed's DLB drug shows promise in phase 2b trial | 3 | Investing.com | ||
| CERVOMED Aktie jetzt für 0€ handeln | |||||
| 02.12.25 | CervoMed Inc.: CervoMed Presents New Plasma Biomarker Data That Indicates Neflamapimod Broadly Improves Neuroinflammation and Neurodegeneration in Dementia with Lewy Bodies (DLB) | 2 | GlobeNewswire (USA) | ||
| 10.11.25 | CervoMed reports Q3 results | 3 | Seeking Alpha | ||
| 07.11.25 | CervoMed Inc. - S-8, Securities to be offered to employees in employee benefit plans | - | SEC Filings | ||
| 07.11.25 | CervoMed Inc. - 10-Q, Quarterly Report | 3 | SEC Filings | ||
| 04.11.25 | CervoMed-Aktie legt nach positivem FDA-Feedback zu Phase-3-Studiendesign zu | 11 | Investing.com Deutsch | ||
| 04.11.25 | CervoMed Inc. - 8-K, Current Report | - | SEC Filings | ||
| 28.10.25 | CervoMed appoints McKinsey veteran David Quigley to board of directors | 3 | Investing.com | ||
| 22.10.25 | CervoMed Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 08.10.25 | CervoMed Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 08.10.25 | CervoMed Drug Cut Dementia Progression Risk By 75% In Trial | 7 | Benzinga.com | ||
| 08.10.25 | CervoMed stock soars after positive dementia treatment trial data | 19 | Investing.com | ||
| 08.10.25 | CervoMed: Aktie legt nach positiven Studiendaten zu Demenz-Wirkstoff deutlich zu | 1 | Investing.com Deutsch | ||
| 08.10.25 | CervoMed Inc.: CervoMed Announces New Data from Phase 2b Trial Demonstrating Neflamapimod's Potential as a Treatment for Dementia with Lewy Bodies | 147 | GlobeNewswire (Europe) | Significant improvement relative to placebo on primary outcome measure, change in Clinical Dementia Rating Sum of Boxes (CDR-SB), demonstrated in a within-subject analysis in participants with low... ► Artikel lesen | |
| 07.10.25 | CervoMed beruft Matthew Winton zum Chief Commercial and Business Officer | 2 | Investing.com Deutsch | ||
| 07.10.25 | CervoMed appoints Matthew Winton as chief commercial and business officer | 11 | Investing.com |
1 2 Weiter >>
27 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 90,40 | +0,17 % | BioNTech- und Nvidia-Aktien verkauft, Medline und Alphabet rein: Erste 13F-Filings sind da! | Der britische Vermögensverwalter Baillie Gifford hat als einer der ersten sein 13F-Filing für das 4. Quartal abgegeben. Unter den großen Namen im Portfolio gibt es ein paar spannende Veränderungen.... ► Artikel lesen | |
| EVOTEC | 6,148 | +0,79 % | Zink-Boom, Turnaround, Biotech-Wachstum: So profitieren Sie mit Pasinex Resources, Puma und Evotec | In volatilen Märkten suchen Anleger nach außergewöhnlichen Chancen. Drei Unternehmen stechen dabei heraus. Ein Rohstoffunternehmen mit außergewöhnlichen Zink-Projekten, ein Sportartikelhersteller im... ► Artikel lesen | |
| BB BIOTECH | 50,60 | -0,78 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| MEDIGENE | 0,042 | -8,26 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 43,170 | -0,70 % | Diagnostikkonzern Qiagen überrascht im Schlussquartal - Weiteres Wachstum 2026 | VENLO/HILDEN (dpa-AFX) - Qiagen hat 2025 von einer anziehenden Nachfrage nach seinen Kernprodukten profitiert. Dabei lief das Schlussquartal besser als vom Labordienstleister und Diagnostikkonzern... ► Artikel lesen | |
| MODERNA | 34,650 | -0,07 % | Arbutus jumps on ruling in patent dispute with Moderna | ||
| PAION | 0,200 | +8,11 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WIRD GEPRUEFT | Fuer folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wird ein Mistrade-Antrag geprueft:ISIN Datum Zeit Volumen Preis Fair Value (AS)DE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 3,986 | +0,61 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| AMGEN | 325,15 | +0,06 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| EPIGENOMICS | 0,870 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 6,910 | -0,80 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| BIOGEN | 164,25 | +3,79 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,420 | -1,22 % | PTA-news: Biofrontera AG: Biofrontera berichtet über die Ergebnisse der ersten neun Monate des Jahres 2025 | DJ PTA-News: Biofrontera AG: BIOFRONTERA BERICHTET ÜBER DIE ERGEBNISSE DER ERSTEN NEUN MONATE DES JAHRES 2025
Unternehmensmitteilung für den Kapitalmarkt
Biofrontera AG: BIOFRONTERA... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,910 | 0,00 % | HEIDELBERG PHARMA AG zeigt klare Stärke im Trend | ||
| CRISPR THERAPEUTICS | 41,200 | -0,48 % | Morningstar, Inc.: Morningstar Completes Acquisition of CRSP and Extends Relationship with Vanguard | The CRSP integration unites two trusted sources of market insight, reinforcing a shared commitment to transparency, quality, and investor-focused solutions and solidifying Morningstar's position... ► Artikel lesen |
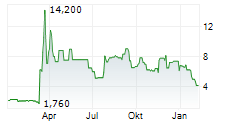
CERVOMED INC gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 4,020 Euro und damit -11,06 % im Minus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu CERVOMED INC finden Sie auf Wallstreet Online.