| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
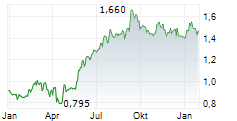
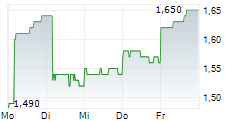
| 1,630 | 1,690 | 07.02. | |
| 1,660 | 1,720 | 06.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| CHINA MEDICAL SYSTEM Aktie jetzt für 0€ handeln | |||||
| Di | CMS (00867): VOLUNTARY AND BUSINESS UPDATE ANNOUNCEMENT APPROVAL OF DRUG CLINICAL TRIALS FOR COMPLEMENT - MEDIATED KIDNEY DISEASE INDICATION OF INNOVATIVE ... | 3 | HKEx | ||
| 30.01. | China Medical System Holdings Ltd.: CMS (867.HK/8A8.SG): Ruxolitinib Phosphate Cream Obtained China NDA Approval, Becoming The First and Only Targeted Drug for Vitiligo in China | 210 | GlobeNewswire (Europe) | SHENZHEN, CHINA, Jan. 30, 2026 (GLOBE NEWSWIRE) -- China Medical System Holdings Limited ("CMS" or the "Group") is pleased to announce that its subsidiary, Dermavon Holdings Limited ("Dermavon", an... ► Artikel lesen | |
| 30.01. | CMS (00867): VOLUNTARY AND BUSINESS UPDATE ANNOUNCEMENT APPROVAL OF DRUG CLINICAL TRIALS FOR PAROXYSMAL NOCTURNAL HEMOGLOBINURIA INDICATION OF INNOVATIVE ... | 2 | HKEx | ||
| 30.01. | CMS (00867): VOLUNTARY AND BUSINESS UPDATE ANNOUNCEMENT NEW DRUG APPLICATION OF RUXOLITINIB PHOSPHATE CREAM APPROVED IN CHINA THE FIRST AND ONLY TARGETED ... | 1 | HKEx | ||
| 22.01. | CMS removes OSA coverage for Inspire Medical Systems reimbursement uplift | 2 | Investing.com | ||
| 15.12.25 | China Medical System Holdings Ltd.: CMS (867.HK/8A8.SG): Innovative Drug Oral JAK1 Inhibitor Povorcitinib Has Been Included in the List of Breakthrough Therapeutic Drugs in China | 2 | GlobeNewswire (USA) | ||
| 15.12.25 | CMS (00867): VOLUNTARY AND BUSINESS UPDATE ANNOUNCEMENT INNOVATIVE DRUG ORAL SMALL-MOLECULE JAK1 INHIBITOR POVORCITINIB INCLUDED IN THE LIST OF BREAKTHROUGH ... | 1 | HKEx | ||
| 11.12.25 | China Medical System Holdings Ltd.: CMS (867/8A8): NDA of Innovative Drug Y-3 for Injection for Acute Ischemic Stroke Accepted in China | 2 | GlobeNewswire (USA) | ||
| 11.12.25 | CMS (00867): VOLUNTARY AND BUSINESS UPDATE ANNOUNCEMENT NEW DRUG APPLICATION OF CLASS 1 INNOVATIVE DRUG Y-3 FOR INJECTION FOR ACUTE ISCHEMIC STROKE ACCEPTED ... | 1 | HKEx | ||
| 31.10.25 | CHINA MEDICAL SYSTEM HOLDINGS LIMITED: GENERAL ANNOUNCEMENT::MONTHLY RETURN OF EQUITY ISSUER ON MOVEMENTS IN SECURITIES FOR THE MONTH ENDED 31 OCTOBER 2025 | - | SGX Company Announcements | ||
| 30.10.25 | China Medical System Holdings Ltd.: CMS (867.HK; 8A8.SG) NDA for AD Indication of Long-acting Anti-IL-4Ra Humanized Monoclonal Antibody Injection MG-K10 Accepted in China | 1 | GlobeNewswire (USA) | ||
| 30.10.25 | CMS (00867): VOLUNTARY AND BUSINESS UPDATE ANNOUNCEMENT NEW DRUG APPLICATION FOR ATOPIC DERMATITIS INDICATION OF LONG-ACTING ANTI-IL-4RA HUMANIZED MONOCLONAL ... | - | HKEx | ||
| 27.10.25 | China Medical System Holdings Ltd.: CMS (867.HK; 8A8.SG) Signed A Distribution Agreement for Ophthalmic Drugs Lucentis and Beovu | 1 | GlobeNewswire (USA) | ||
| 27.10.25 | CMS (00867): VOLUNTARY AND BUSINESS UPDATE ANNOUNCEMENT SIGNING A DISTRIBUTION AGREEMENT FOR OPHTHALMIC DRUGS LUCENTIS AND BEOVU | - | HKEx | ||
| 30.09.25 | CMS (00867): VOLUNTARY AND BUSINESS UPDATE ANNOUNCEMENT APPROVAL OF DRUG CLINICAL TRIALS FOR ADDITIONAL CHRONIC OBSTRUCTIVE PULMONARY DISEASE INDICATION ... | - | HKEx | ||
| 28.09.25 | China Medical System Holdings Ltd. (867.HK; 8A8.SG) Positive Results from China Phase 3 Clinical Trial of Innovative Drug Ruxolitinib Cream with AD Indication | 771 | GlobeNewswire (Europe) | SHENZHEN, CHINA, Sept. 28, 2025 (GLOBE NEWSWIRE) -- China Medical System Holdings Limited ("CMS") is pleased to announce that its subsidiaries, Dermavon Holdings Limited ("Dermavon", an innovative... ► Artikel lesen | |
| 26.09.25 | CMS (00867): VOLUNTARY AND BUSINESS UPDATE ANNOUNCEMENT POSITIVE RESULTS FROM PHASE 3 CLINICAL TRIAL OF RUXOLITINIB CREAM IN CHINESE PATIENTS WITH ATOPIC ... | 3 | HKEx | ||
| 26.09.25 | CMS (00867): VOLUNTARY AND BUSINESS UPDATE ANNOUNCEMENT APPROVAL OF DRUG CLINICAL TRIALS FOR UTERINE FIBROIDS INDICATION OF INNOVATIVE DRUG GNRH RECEPTOR ... | - | HKEx | ||
| 23.09.25 | China Medical System Holdings Ltd. (867.HK; 8A8.SG) Signed Collaboration Agreements for Two Innovative Biologics Used for Passive Immunization Against Tetanus and Rabies | 424 | GlobeNewswire (Europe) | SHENZHEN, CHINA, Sept. 23, 2025 (GLOBE NEWSWIRE) -- China Medical System Holdings Limited ("CMS" or the "Group") is pleased to announce that on 22 September 2025, the Group through its subsidiaries... ► Artikel lesen | |
| 22.09.25 | CMS (00867): VOLUNTARY AND BUSINESS UPDATE ANNOUNCEMENT SIGNING COLLABORATION AGREEMENTS FOR CLASS 1 INNOVATIVE BIOLOGIC RECOMBINANT HUMANIZED ANTI-TETANUS ... | 1 | HKEx |
1 2 Weiter >>
35 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| FRESENIUS | 49,760 | +1,06 % | JEFFERIES stuft Fresenius SE auf 'Buy' | NEW YORK (dpa-AFX Analyser) - Das Analysehaus Jefferies hat die Einstufung für Fresenius mit einem Kursziel von 55 Euro auf "Buy" belassen. Die Bad Homburger seien in Verjüngungskur, und der Markt preise... ► Artikel lesen | |
| FRESENIUS MEDICAL CARE | 40,640 | +0,40 % | Fresenius Medical Care: Ein Neuanfang nach turbulenten Zeiten? | ||
| SIEMENS HEALTHINEERS | 41,400 | -2,82 % | HV-Termine: Hauptversammlungen bei Adv. Blockchain, Aurubis, Fortec, Schott, Siemens, Healthineers, Stabilus, TUI | Einmal jährlich müssen sich Aufsichtsrat und Vorstand einer Gesellschaft den Aktionären stellen: Die Hauptversammlung ist das höchste Organ einer Aktiengesellschaft und vergleichbarer Unternehmens-Formen.... ► Artikel lesen | |
| CARL ZEISS MEDITEC | 27,520 | +0,66 % | BARCLAYS stuft CARL ZEISS MEDITEC AG auf 'Equal Weight' | LONDON (dpa-AFX Analyser) - Die britische Investmentbank Barclays hat das Kursziel für Carl Zeiss Meditec von 49 auf 32 Euro gesenkt und die Aktien von "Overweight" auf "Equal Weight" abgestuft. Nur... ► Artikel lesen | |
| ECKERT & ZIEGLER | 15,440 | +1,65 % | BERENBERG stuft ECKERT & ZIEGLER auf 'Buy' | HAMBURG (dpa-AFX Analyser) - Die Privatbank Berenberg hat die Einstufung für Eckert & Ziegler mit einem Kursziel von 24 Euro auf "Buy" belassen. Die vorläufigen Zahlen für 2025 hätten beim Umsatz und... ► Artikel lesen | |
| UNITEDHEALTH | 232,15 | -0,77 % | UnitedHealth: Das sieht gar nicht gut aus | Der enttäuschende Ausblick, den UnitedHealth dem Kapitalmarkt vor Kurzem an die Hand gegeben hat, und maue Aussichten für 2027 hinterlassen weitere Spuren im Aktienkurs des größten US-Krankenversicherers.... ► Artikel lesen | |
| DRAEGERWERK | 86,30 | -0,46 % | DAX wartet auf Fed - Airbus, Bayer, Drägerwerk, Infineon, SUSS Microtec, Wacker Chemie im ... | Am deutschen Aktienmarkt herrscht zu Handelsbeginn wenig Bewegung - die Anleger warten gespannt auf die nächste Notenbankentscheidung in den USA heute Abend. Am Vortag scheiterte der DAX zudem an der... ► Artikel lesen | |
| MEDTRONIC | 86,88 | -0,17 % | AKTIONÄR-Tipp Medtronic: Übernahme | Viel Bewegung an der Börse: Bei zahlreichen Wachstumstiteln wurden in den zurückliegenden Tagen teils deutlich Gewinne vom Tisch genommen. Der Fokus der Anleger richtet sich zusehends wieder auf defensivere... ► Artikel lesen | |
| GERRESHEIMER | 25,660 | +1,99 % | JEFFERIES stuft GERRESHEIMER AG auf 'Buy' | NEW YORK (dpa-AFX Analyser) - Das Analysehaus Jefferies hat die Einstufung für Gerresheimer mit einem Kursziel von 34,10 Euro auf "Buy" belassen. Analyst James Vane-Tempest zählt den Spezialverpackungshersteller... ► Artikel lesen | |
| INTUITIVE SURGICAL | 413,35 | +0,02 % | INTUITIVE SURGICAL INC - 10-K, Annual Report | ||
| THERMO FISHER | 460,70 | +0,35 % | Thermo Fisher to Close Franklin, Mass. Site, Cut Up to 80 Jobs | ||
| TELADOC HEALTH | 4,240 | -0,05 % | Teladoc Health, Inc.: Teladoc Health to Announce Fourth Quarter 2025 Financial Results | ||
| STRATEC | 21,050 | +0,72 % | Stratec: Experten rechnen mit konservativem Ausblick | Das vierte Quartal 2024 war bei Stratec besonders stark. Dieses Niveau dürfte die Gesellschaft Ende 2025 nicht erreicht haben. So rechnen die Analysten der Deutschen Bank auch mit einem Minus von 5... ► Artikel lesen | |
| RHOEN-KLINIKUM | 13,200 | +1,54 % | EQS-Adhoc: RHÖN-KLINIKUM Aktiengesellschaft: RHÖN-KLINIKUM passt die EBITDA-Prognose für das Geschäftsjahr 2025 an | EQS-Ad-hoc: RHÖN-KLINIKUM Aktiengesellschaft / Schlagwort(e): Prognoseänderung
RHÖN-KLINIKUM Aktiengesellschaft passt die EBITDA-Prognose für das Geschäftsjahr 2025 an... ► Artikel lesen | |
| PROCEPT BIOROBOTICS | 28,740 | +4,89 % | PROCEPT BioRobotics Reports Third Quarter 2025 Financial Results and Issues 2026 Revenue Guidance | SAN JOSE, Calif., Nov. 04, 2025 (GLOBE NEWSWIRE) -- PROCEPT BioRobotics® Corporation (Nasdaq: PRCT) (the "Company"), a surgical robotics company focused on advancing patient care by developing transformative... ► Artikel lesen |
Das Unternehmen CHINA MEDICAL SYSTEM HOLDINGS LTD kann der Branche Gesundheitswesen zugeordnet werden. Der aktuelle Kurs liegt bei 1,660 Euro und damit 0,00 % im Plus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu CHINA MEDICAL SYSTEM HOLDINGS LTD finden Sie auf Wallstreet Online.