| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 0,868 | 0,904 | 19:41 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 27.03. | CROSSJECT reports financial results for 2024 | 107 | GlobeNewswire (Europe) | Press Release CROSSJECT reports financial results for 2024 Continued satisfactory production of regulatory batches with a view to filing in Q2 2025.Recruitment of Tony Tipton as Chief Operating Officer... ► Artikel lesen | |
| 25.03. | CROSSJECT updates on its collaboration with ETON PHARMACEUTICALS, Inc. and the ZENEO adrenal insufficiency program | 106 | GlobeNewswire (Europe) | Press Release CROSSJECT updates on its collaboration with ETON PHARMACEUTICALS, Inc. and the ZENEO® adrenal insufficiency program CROSSJECT enhanced its collaboration with Eton Pharmaceuticals,... ► Artikel lesen | |
| 18.03. | CROSSJECT achieves key ZEPIZURE manufacturing batch stability milestones | 1 | GlobeNewswire (USA) | ||
| 12.02. | Crossject announces the reappointment of Patrick Alexandre and Isabelle Liebschutz as members of new, streamlined Executive Board | 131 | GlobeNewswire (Europe) | Dijon, FRANCE, February 12, 2025 - 0730 (CET) - Crossject (ISIN: FR0011716265; Euronext Growth: ALCJ), a specialty pharma company developing medicines harnessing its unique, award-winning needle-free... ► Artikel lesen | |
| 19.12.24 | Crossject confirms ZEPIZURE supply chain readiness with another successful ISO audit for Quality Management System | 119 | GlobeNewswire (Europe) | Crossject successfully passes another ISO 13485 audit for sites in Dijon and Gray (France)Company is ramping up supply chain to prepare for commercialization in U.S. Dijon, France, December 19, 2024... ► Artikel lesen | |
| 11.12.24 | Crossject announces the pricing of its reserved capital increase and warrants issuance for an aggregate amount of EUR 7.2M | 2 | GlobeNewswire (USA) | ||
| 10.12.24 | Crossject launches reserved capital increase and warrants issuance for an aggregate amount of at least €7m | 1 | GlobeNewswire (USA) | ||
| CROSSJECT Aktie jetzt für 0€ handeln | |||||
| 09.12.24 | Crossject and the U.S. Department of Defense Relaunch Joint Research on Needle-free Autoinjectors, Extending Their Cooperative Research and Development Agreement | 1 | GlobeNewswire (USA) | ||
| 13.11.24 | Crossject's ZENEO Auto-Injector's usability further demonstrated in extreme HAZMAT conditions | 1 | GlobeNewswire (USA) | ||
| 22.10.24 | Crossject reports strong manufacturing progress with its epilepsy rescue therapy ZEPIZURE ahead of filing U.S. Emergency Use Authorization | 135 | GlobeNewswire (Europe) | Product batch announced in July yielded new regulatory stability data. These results will directly support the requested 6-month stability data in the first quarter of 2025Crossject expects to file... ► Artikel lesen | |
| 23.09.24 | Crossject reports financial results and business highlights for the first six months of 2024 | 255 | GlobeNewswire (Europe) | Available cash €5.95 million, up from €2.3 million on December 31, 2023Reports stable investments in R&D and consolidation of operating income from BARDACompany on track to successfully file for the... ► Artikel lesen | |
| 19.08.24 | Crossject appoints Tony Tipton as U.S. Chief Operating Officer in Preparation for Commercialization of ZEPIZURE Epilepsy Rescue Treatment | 262 | GlobeNewswire (Europe) | Press Release Crossject appoints Tony Tipton as U.S. Chief Operating Officer in Preparation for Commercialization of ZEPIZURE® Epilepsy Rescue Treatment Experienced executive to lead preparations... ► Artikel lesen | |
| 18.07.24 | Crossject achieves key ZEPIZURE manufacturing milestone | 184 | GlobeNewswire (Europe) |
Successful completion of an additional Registration Batch of ZEPIZURE® at new manufacturing siteMilestone complements the satisfying results from previous batches under stability studies, and is... ► Artikel lesen | |
| 10.07.24 | Crossject is awarded a 6.9 million euros financing from the French Government, as part of the France 2030 innovation plan, to accelerate the development of ZENEO Epinephrine | 201 | GlobeNewswire (Europe) | Key contribution to the acceleration of the development of ZENEO® EpinephrineFinancial support includes mainly grants, as well as subsidized loansFrance 2030 Plan dedicated to supporting French companies... ► Artikel lesen | |
| 26.06.24 | Crossject elaborates on ZEPIZURE potential in light of landmark RAMPART study and its own, recently published, bioequivalence study | 250 | GlobeNewswire (Europe) | Landmark RAMPART study established midazolam intramuscular (IM) injection as a standard of care in the pre-hospital emergency management of epilepsy crises compared to traditional benzodiazepine... ► Artikel lesen | |
| 12.06.24 | Crossject appoints Dan Chiche, MD as Chief Medical Officer North America | 255 | GlobeNewswire (Europe) | Brings extensive experience in drug development in the U.S and internationally and in maximizing value of medical productsReinforces leadership team and expands presence in North AmericaSupports processes... ► Artikel lesen | |
| 14.05.24 | XFRA CAPITAL ADJUSTMENT INFORMATION - 14.05.2024 | 801 | Xetra Newsboard | Das Instrument 3W1 SE0000236382 CONCEJO AB (PUBL) B SK 5 EQUITY wird ex Kapitalmassnahme gehandelt am 14.05.2024 The instrument 3W1 SE0000236382 CONCEJO AB (PUBL) B SK 5 EQUITY is traded ex capital... ► Artikel lesen | |
| 25.04.24 | Crossject reports audited financial results for 2023 | 243 | GlobeNewswire (Europe) | Dijon, France April 25, 2024 -- Crossject (ISIN: FR0011716265; Euronext: ALCJ), a specialty pharmaceuticals company developing products for emergency situations harnessing its proprietary needle-free... ► Artikel lesen |
18 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| FRESENIUS | 39,850 | +0,94 % | Fresenius SE Aktie: Leichte Erholung im Fokus | Fresenius notiert nahe dem Jahreshoch mit soliden technischen und fundamentalen Signalen. Analysten sehen weiteres Kurspotenzial trotz hoher Verschuldung. Die Fresenius-Aktie zeigt sich am Donnerstag... ► Artikel lesen | |
| INTUITIVE SURGICAL | 447,25 | +3,87 % | Intuitive Surgical Aktie: Roboter-Chirurgie mit gemischtem Bild | Intuitive Surgical meldet starkes Umsatz- und Verfahrenswachstum, doch Handelszölle drücken die Margen. Wie reagiert der Markt auf die gemischten Signale? Der Medizintechnik-Konzern Intuitive Surgical... ► Artikel lesen | |
| SERNOVA BIOTHERAPEUTICS | 0,133 | -1,48 % | Sernova Biotherapeutics Inc: Sernova closes $4-million term loan from Navigate | ||
| FAMICORD | 4,200 | +3,45 % | EQS-AFR: FamiCord AG: Vorabbekanntmachung über die Veröffentlichung von Finanzberichten gemäß §§ 114, 115, 117 WpHG | EQS Vorabbekanntmachung Finanzberichte: FamiCord AG
/ Vorabbekanntmachung über die Veröffentlichung von Rechnungslegungsberichten
FamiCord AG: Vorabbekanntmachung über die Veröffentlichung... ► Artikel lesen | |
| GERATHERM MEDICAL | 2,860 | +2,88 % | Geratherm Medical Aktie: Ruhige Zeiten angekündigt? | Das Medizintechnikunternehmen passt Produktionskapazitäten für klinische Thermometer bei fortgesetzter Marktschwäche an, während die Aktie trotz Volatilität zuletzt zulegte. Die Geratherm Medical AG... ► Artikel lesen | |
| ALIGN TECHNOLOGY | 161,40 | +3,10 % | Aktienmarkt: Kurs der Aktie von Align Technology im Minus (134,2835 €) | Die Align Technology-Aktie notiert heute ein wenig leichter. Die Aktie notiert zur Stunde bei 147,07 US-Dollar. An der Börse liegt das Wertpapier von Align Technology aktuell im Minus. Das Papier verbilligte... ► Artikel lesen | |
| NUGEN MEDICAL DEVICES | 0,052 | -6,12 % | Osterlektüre: Die 13.000%-Chance für ihr Depot! | ||
| PLUS THERAPEUTICS | 0,880 | -11,08 % | Plus Therapeutics beruft Kyle Guse in den Vorstand | ||
| CARDINAL HEALTH | 117,90 | -0,59 % | What You Need To Know Ahead of Cardinal Health's Earnings Release | ||
| RESMED | 208,50 | +10,14 % | Guter Tag für ResMed-Aktionäre: Aktienkurs steigt deutlich (199,50 €) | Zu den großen Gewinnern an der Börse zählt heute die ResMed-Aktie . Das Wertpapier steigt deutlich. Eine Steigerung um 5,39 Prozent - mit diesem Wertanstieg gehört das Wertpapier von ResMed aktuell... ► Artikel lesen | |
| DAVITA | 121,05 | -1,38 % | DaVita's acquisition of Brasnefro approved with divestiture plan | ||
| ATOSSA THERAPEUTICS | 0,639 | -1,69 % | Atossa Therapeutics, Inc.: Atossa Therapeutics Announces Issuance of U.S. Patent No. 12,275,684, Further Strengthening (Z)-endoxifen Portfolio | SEATTLE, April 22, 2025 (GLOBE NEWSWIRE) -- Atossa Therapeutics, Inc. (Nasdaq: ATOS) ("Atossa" or the "Company"), a clinical-stage biopharmaceutical company developing innovative medicines for breast... ► Artikel lesen | |
| MEDICLIN | 2,920 | -2,01 % | EQS-HV: MEDICLIN Aktiengesellschaft: Bekanntmachung der Einberufung zur Hauptversammlung am 04.06.2025 in Bad Neustadt a. d. Saale mit dem Ziel der europaweiten Verbreitung gemäß §121 AktG | EQS-News: MEDICLIN Aktiengesellschaft
/ Bekanntmachung der Einberufung zur Hauptversammlung
MEDICLIN Aktiengesellschaft: Bekanntmachung der Einberufung zur Hauptversammlung am... ► Artikel lesen | |
| UNIDOC HEALTH | 0,203 | -0,49 % | UniDoc Health Corp.: UniDoc Announces LIFE Offering | THIS NEWS RELEASE IS NOT FOR DISTRIBUTION TO U.S. NEWSWIRE SERVICES OR FOR DISSEMINATION IN THE UNITED STATES VANCOUVER, BC / ACCESS Newswire / April 4, 2025 / UniDoc Health Corp. (CSE:UDOC) ("UniDoc"... ► Artikel lesen | |
| EVOFEM BIOSCIENCES | - | - | Evofem Biosciences, Inc.: Evofem Biosciences Announces Financial Results for the Third Quarter of 2024 | - Improved loss from operations by 31% -
- Acquired SOLOSEC, a commercially attractive, single-dose oral antibiotic FDA-approved to treat two pervasive sexual health... ► Artikel lesen |
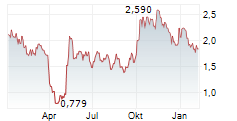
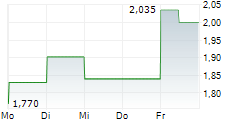
CROSSJECT SA gehört der Branche Gesundheitswesen an. Mit einer aktuellen Kursveränderung von 0,000 (0,00 %) liegt der Kurs bei 0,930 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und .
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu CROSSJECT SA finden Sie auf Wallstreet Online.