- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| DERMATA THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| Di | Dermata Therapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 27.01. | Dermata Therapeutics, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 20.01. | Dermata receives Australian patent for Spongilla acne treatment | 2 | Investing.com | ||
| 20.01. | Dermata Therapeutics: Dermata Receives Notice of Grant of Patent for Next-Generation Acne Treatment by Australian Patent Office | 259 | ACCESS Newswire | This patent grant follows the Company's U.S. issued patent, covering its Spongilla technology combination as a method to topically treat acneDermata expects to launch a new once-weekly, over-the-counter... ► Artikel lesen | |
| 29.12.25 | Dermata Therapeutics Announces Closing of up to $12.4 Million Private Placement Priced At-The-Market Under Nasdaq Rules | 368 | ACCESS Newswire | $4.1 million upfront with up to approximately $8.3 million of potential additional gross proceeds upon the exercise in full of warrants SAN DIEGO, CA / ACCESS Newswire / December 29, 2025 / Dermata... ► Artikel lesen | |
| 29.12.25 | Dermata Therapeutics, Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 26.12.25 | Dermata Therapeutics Announces A Private Placement Valued At Up To $12.4 Mln | 2 | RTTNews | ||
| 24.12.25 | Dermata Vaults on Share Sale | 3 | Baystreet.ca | ||
| 24.12.25 | Privatplatzierung beflügelt Aktie von Dermata Therapeutics | 2 | Investing.com Deutsch | ||
| 24.12.25 | Dermata Therapeutics announces up to $12.4 million private placement | 1 | Seeking Alpha | ||
| 24.12.25 | Dermata Therapeutics Announces up to $12.4 Million Private Placement Priced At-The-Market Under Nasdaq Rules | 452 | ACCESS Newswire | $4.1 million upfront with up to approximately $8.3 million of potential additional gross proceeds upon the exercise in full of warrants SAN DIEGO, CA / ACCESS Newswire / December 24, 2025 / Dermata... ► Artikel lesen | |
| 04.12.25 | Dermata Therapeutics: A New Realm of Skincare is Coming: Dermata Teases New OTC Brand Identity | 265 | ACCESS Newswire | SAN DIEGO, CA / ACCESS Newswire / December 4, 2025 / Dermata Therapeutics, Inc. (NASDAQ:DRMA)(NASDAQ:DRMAW) ("Dermata" or the "Company"), a science-driven leader in dermatologic solutions, today announced... ► Artikel lesen | |
| 14.11.25 | Dermata Therapeutics GAAP EPS of -$1.65 | 6 | Seeking Alpha | ||
| 14.11.25 | Dermata Therapeutics Provides Corporate Update and Reports Third Quarter 2025 Financial Results | 340 | ACCESS Newswire | - Dermata announced a strategic pivot to develop and commercialize over-the-counter (OTC) skin care treatments -- Dermata plans to launch its first OTC product, a once weekly acne kit with its Spongilla... ► Artikel lesen | |
| 07.11.25 | Dermata Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 02.10.25 | Australian patent office accepts Dermata's acne treatment patent | 3 | Investing.com | ||
| 02.10.25 | Dermata Therapeutics: Dermata Announces Acceptance of Patent Application for Next-Generation Acne Treatment by Australian Patent Office | 286 | ACCESS Newswire | - This acceptance follow's Dermata's U.S. issued patent covering its Spongilla technology combination as a method to topically treat acne -- Dermata expects to launch a new once-weekly, Over-the-Counter... ► Artikel lesen | |
| 17.09.25 | Dermata Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 17.09.25 | Dermata Therapeutics Announces Presentation of Abstract at the European Academy of Dermatology and Venereology Congress 2025 | 391 | ACCESS Newswire | - Abstract highlights additional primary and secondary data from Phase 3 STAR-1 clinical study of XYNGARI for the treatment of moderate-to-severe acne - SAN DIEGO, CA / ACCESS Newswire / September 17... ► Artikel lesen | |
| 10.09.25 | Dermata Therapeutics Announces Strategic Pivot to Over-the-Counter Skin Care Treatments | 327 | ACCESS Newswire | SAN DIEGO, CA / ACCESS Newswire / September 10, 2025 / Dermata Therapeutics, Inc. (Nasdaq:DRMA)(Nasdaq:DRMAW) ("Dermata" or the "Company"), a science-driven leader in dermatologic solutions, today announced... ► Artikel lesen |
1 2 Weiter >>
33 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 90,40 | +0,17 % | BioNTech- und Nvidia-Aktien verkauft, Medline und Alphabet rein: Erste 13F-Filings sind da! | Der britische Vermögensverwalter Baillie Gifford hat als einer der ersten sein 13F-Filing für das 4. Quartal abgegeben. Unter den großen Namen im Portfolio gibt es ein paar spannende Veränderungen.... ► Artikel lesen | |
| EVOTEC | 6,148 | +0,79 % | SDAX: Evotec-Aktie schießt zweistellig nach oben | Eine Kaufempfehlung von Berenberg entfacht neuen Optimismus bei Evotec und katapultiert die Biotech-Aktie an die Indexspitze. Die Aktie der Evotec hat am Dienstagmittag ein Kursfeuerwerk gezündet und... ► Artikel lesen | |
| BB BIOTECH | 50,60 | -0,78 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| QIAGEN | 43,170 | -0,70 % | JEFFERIES stuft QIAGEN NV auf 'Buy' | NEW YORK (dpa-AFX Analyser) - Das Analysehaus Jefferies hat die Einstufung für Qiagen mit einem Kursziel von 57 US-Dollar auf "Buy" belassen. Umsatz und Ergebnis des vierten Quartals hätten die Erwartungen... ► Artikel lesen | |
| PAION | 0,200 | +8,11 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WIRD GEPRUEFT | Fuer folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wird ein Mistrade-Antrag geprueft:ISIN Datum Zeit Volumen Preis Fair Value (AS)DE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 3,986 | +0,61 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| NOVAVAX | 6,910 | -0,80 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| STRYKER | 302,10 | -0,33 % | Stryker Corporation: Stryker declares an $0.88 per share quarterly dividend | Portage, Michigan, Feb. 04, 2026 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) announced that its Board of Directors has declared a quarterly dividend of $0.88 per share payable April 30, 2026, to shareholders... ► Artikel lesen | |
| BIOGEN | 164,25 | +3,79 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,420 | -1,22 % | PTA-news: Biofrontera AG: Biofrontera berichtet über die Ergebnisse der ersten neun Monate des Jahres 2025 | DJ PTA-News: Biofrontera AG: BIOFRONTERA BERICHTET ÜBER DIE ERGEBNISSE DER ERSTEN NEUN MONATE DES JAHRES 2025
Unternehmensmitteilung für den Kapitalmarkt
Biofrontera AG: BIOFRONTERA... ► Artikel lesen | |
| CRISPR THERAPEUTICS | 41,200 | -0,48 % | Morningstar, Inc.: Morningstar Completes Acquisition of CRSP and Extends Relationship with Vanguard | The CRSP integration unites two trusted sources of market insight, reinforcing a shared commitment to transparency, quality, and investor-focused solutions and solidifying Morningstar's position... ► Artikel lesen | |
| CORE ONE LABS | - | - | XFRA DELETION OF INSTRUMENTS FROM BOERSE FRANKFURT - 22.09.2025 | The following instruments on Boerse Frankfurt do have their last trading day on 22.09.2025Die folgenden Instrumente in Boerse Frankfurt haben ihren letzten Handelstag am 22.09.2025ISIN NameAU000000PEK2 PEAK... ► Artikel lesen | |
| REGENERON PHARMACEUTICALS | 662,80 | -0,27 % | REGENERON PHARMACEUTICALS, INC. - 10-K, Annual Report | ||
| BIOXXMED | 1,328 | 0,00 % | XFRA NEW INSTRUMENTS AVAILABLE ON 14.01.2026 | The following instruments on XETRA do have their first trading 14.01.2026 Die folgenden Instrumente in XETRA haben ihren ersten Handelstag 14.01.2026
Aktien
1 MHY3130D1013 Heidmar Maritime Hldgs.... ► Artikel lesen | |
| BIONXT SOLUTIONS | 0,387 | +2,38 % | BioNxt sichert sich innovative Chaperon-Technologie zur Verbesserung der oralen Dünnfilm-Wirkstoffverabreichung | VANCOUVER, BC / ACCESS Newswire /
5. Februar 2026 - BioNxt Solutions Inc. ("BioNxt" oder das "Unternehmen") (CSE: BNXT) (OTCQB: BNXTF) (FSE: BXT), ein biowissenschaftliches
Innovationsunternehmen... ► Artikel lesen |
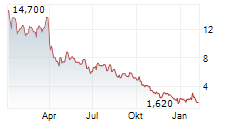
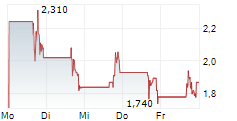
DERMATA THERAPEUTICS INC gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von +0,100 (+5,65 %) liegt der Kurs bei 1,870 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu DERMATA THERAPEUTICS INC finden Sie auf Wallstreet Online.