- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mo | DR REDDYS LABORATORIES LTD - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 30.01. | Coya Therapeutics Sets $11.1 Million Private Placement Led by Dr. Reddy's And Greenlight Capital | 2 | pulse2.com | ||
| 30.01. | Coya Therapeutics raises $11.1M in private placement led by Dr. Reddy's | 7 | Seeking Alpha | ||
| 22.01. | Dr. Reddy's Q3 Earnings Match Estimates, Revenues Rise Y/Y | 7 | Zacks | ||
| 22.01. | Gainers & Losers: IIFL Finance, Dr Reddy's among 7 stocks that moved most on Thursday | 2 | Times of India | ||
| 22.01. | Dr. Reddy's Labs ADR: EPS übertrifft Schätzungen um 14,36 $ - Umsatz besser als erwartet | 3 | Investing.com Deutsch | ||
| 22.01. | Dr Reddy's shares surge 5% despite 14% fall in profit? Why brokerages are bullish | 5 | ZEE Business | ||
| DR REDDYS Aktie jetzt für 0€ handeln | |||||
| 22.01. | Dr. Reddy's GAAP EPS of Rs 14.52, revenue of Rs 87.27B | 3 | Seeking Alpha | ||
| 22.01. | Dr Reddy's Lab Q3 PAT drops 14% YoY to Rs 1,210 cr | 2 | capitalmarket.com | ||
| 22.01. | Stock Alert: Eternal, Bajaj Consumer, Dr Reddys Lab, KEI Industries, PNB Housing | 1 | capitalmarket.com | ||
| 21.01. | DR REDDYS LABORATORIES LTD - 6-K, Report of foreign issuer | 2 | SEC Filings | ||
| 21.01. | Dr. Reddy's wins India nod for generic Ozempic | 58 | Seeking Alpha | ||
| 21.01. | Dr. Reddy's Q3 FY26 slides: Emerging markets drive growth amid North America decline | 2 | Investing.com | ||
| 21.01. | Dr Reddy's Labs Q3 profit falls 14%; Check revenue, margin and other key details | 2 | ZEE Business | ||
| 21.01. | Dr. Reddy's Net Profit Declines To Rs 1,210 Crore In Q3, Revenue Rises 4.4% YoY To Rs 8,727 Crore | 1 | The Free Press Journal | ||
| 21.01. | Dr Reddy's Laboratories Ltd. Bottom Line Retreats In Q3 | - | RTTNews | ||
| 20.01. | Dr. Reddy's Q3 Earnings Preview | 2 | Seeking Alpha | ||
| 09.01. | DR REDDYS LABORATORIES LTD - 6-K, Report of foreign issuer | 2 | SEC Filings | ||
| 01.01. | Dr Reddy's Swiss subsidiary gets USFDA CRL for biosimilar AVT03 | 5 | capitalmarket.com | ||
| 01.01. | Stock Alert: Canara Bank, NBCC (India), Titagarh Rail Systems, Dr Reddy's Laboratories, Time Technoplast | 1 | capitalmarket.com |
1 2 3 4 5 Weiter >>
81 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 45,800 | +2,20 % | Lösung für Milliarden-Dollar-Problem: MustGrow Biologics validiert Geschäftsmodell - revolutionäre Meldung auch für Bayer und Corteva Agriscience | Raps ist für Kanada fast das, was Erdöl für Saudi-Arabien ist: Ein wirtschaftlicher Motor von gigantischem Ausmaß. Mit einem geschätzten Produktionswert von rund 14 Mrd. CAD im Jahr 2025 ist die gelb... ► Artikel lesen | |
| NOVO NORDISK | 40,310 | +0,01 % | Novo Nordisk Aktie: Aufgepasst, denn es wird spannend! Das Golden Cross rückt näher! | © Foto: 2026 Novo Nordisk A/SDie Novo Nordisk-Aktie steht vor einem entscheidenden charttechnischen Signal. Nach dem brutalen Absturz könnte sich das Blatt wenden. Der Kurs kämpft sich zurück, die wichtigen... ► Artikel lesen | |
| PFIZER | 23,015 | -0,07 % | Pfizer: Gute Zahlen, neue Daten - Kampfansage an Novo Nordisk und Co | Der Pharma-Gigant Pfizer hat am Dienstag die Zahlen für das abgelaufene vierte Quartal respektive Gesamtjahr 2025 veröffentlicht. Diese liegen über dem Markterwartungen. Darüber hinaus veröffentlichten... ► Artikel lesen | |
| NOVARTIS | 132,06 | +0,03 % | Novartis Pharma AG: Novartis erzielte im Jahr 2025 eine Umsatzsteigerung im hohen einstelligen Prozentbereich, eine Kerngewinnmarge von 40% sowie weitere Fortschritte in der Pipeline | Ad-hoc-Mitteilung gemäss Art. 53 KR
Geschäftsjahr
Der Nettoumsatz wuchs um +8% (kWk1, +8% USD), das operative Kernergebnis1 verbesserte sich um +14% (kWk, +12% USD)
Das Umsatzwachstum... ► Artikel lesen | |
| MERCK KGAA | 121,45 | -0,49 % | Merck KGaA: Aktie wird abgestuft | Am 5. März stehen bei Merck die Zahlen zum vierten Quartal an. Dann dürfte es auch eine Prognose für 2026 geben. Die Analysten der Deutschen Bank glauben, dass die Quartalszahlen keine Überraschungen... ► Artikel lesen | |
| GILEAD SCIENCES | 128,98 | -0,05 % | Gilead wins favorable U.S. label update for Yescarta | ||
| AURORA CANNABIS | 2,925 | -0,85 % | Aurora Cannabis Inc.: Aurora Cannabis Announces Fiscal 2026 Third Quarter Results | NASDAQ | TSX: ACB
Expands YoY Total Net Revenue1 by 7% to $94.2 million, with Global Medical Cannabis Net Revenue1 Increasing by 12% to a Record $76.2 million
... ► Artikel lesen | |
| SANOFI | 80,49 | +0,05 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| GSK | 25,300 | +1,12 % | GSK: Besser als erwartet | Der Pharmakonzern konnte ein unerwartet starkes Schlussquartal vorweisen und damit besser abschneiden als gedacht. Der Umsatz von GSK klettert im vergangenen Jahr um 4 % auf knapp 32,7 Mrd. Pfund, währungsbereinigt... ► Artikel lesen | |
| ABBVIE | 189,20 | +0,11 % | Aktien New York Ausblick: Keine Tech-Erholung - Eli Lilly steigen kräftig | NEW YORK (dpa-AFX) - Mit Blick auf den Technologiesektor dürften sich die Investoren an den US-Börsen am Mittwoch zurückhalten. Der Nasdaq 100 Index, der am Vortag deutlich gefallen war, dürfte sich... ► Artikel lesen | |
| CANOPY GROWTH | 0,935 | -0,95 % | Canopy Growth: Treffer ohne Gewinn - Trading-Tipp wird zurückgezogen | ||
| ROCHE | 391,10 | -0,29 % | DZ BANK stuft ROCHE HOLDINGS AG auf 'Kaufen' | FRANKFURT (dpa-AFX Analyser) - Die DZ Bank hat den fairen Aktienwert für Roche von 324 auf 395 Franken angehoben und die Einstufung auf "Kaufen" belassen. "Die Pharma-Sparte hat den großen, nahezu parallelen... ► Artikel lesen | |
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 894,80 | -0,01 % | Novo Nordisk-Aktie stürzt ab! Was macht Eli Lilly? Hochspannung vor Quartalszahlen des US-Konzerns | Der dänische Pharmariese Novo Nordisk steht unter massivem Druck: Umsatzwarnung, Kurssturz und scharfer internationaler Wettbewerb setzen dem Konzern zu. Anleger blicken nun gespannt auf die Zahlen... ► Artikel lesen | |
| MERCK & CO | 103,20 | 0,00 % | Merck & Co: Ausblick enttäuscht - Aktie unter Druck | Der US-amerikanische Pharma-Gigant Merck & Co hat am Dienstag die Zahlen für das abgelaufene vierte Quartal respektive Gesamtjahr 2025 veröffentlicht. Diese sind über den Erwartungen ausgefallen. Allerdings... ► Artikel lesen |
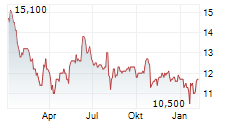
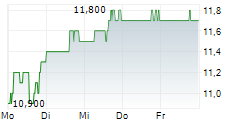
DR REDDYS LABORATORIES LTD ADR gehört der Branche Pharma an. Die relative Kursveränderung beträgt aktuell 0,00 % bei einem Aktienkurs von 11,800 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu DR REDDYS LABORATORIES LTD ADR finden Sie auf Wallstreet Online.