- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Fr | Eisai, Henlius Strike Japan Commercialization Deal for PD-1 Antibody Serplulimab | 3 | Contract Pharma | ||
| Fr | Leqembi starts to deliver for Eisai and Biogen | 13 | pharmaphorum | ||
| Fr | Eisai, Henlius partner on anti-PD-1 antibody in Japan | 2 | Investing.com | ||
| Fr | Eisai and Henlius Enter into Exclusive Commercial License Agreement for Anti-PD-1 Antibody Serplulimab in Japan | 278 | JCN Newswire | TOKYO and SHANGHAI, Feb 6, 2026 - (JCN Newswire) - Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, "Eisai") and Shanghai Henlius Biotech, Inc. (Headquarters: Shanghai, China, CEO: Jason Zhu... ► Artikel lesen | |
| Do | Eisai signs $388m deal for Japanese rights to Henlius' anti-PD-1 mAb | 3 | Pharmaceutical Technology | ||
| Do | Eisai and Henlius Enter into Exclusive Commercial License Agreement for Anti-PD-1 Antibody Serplulimab in Japan | 316 | PR Newswire | TOKYO and SHANGHAI, Feb. 5, 2026 /PRNewswire/ -- Eisai Co., Ltd. ("Eisai") and Shanghai Henlius Biotech, Inc. ("Henlius") today announced the conclusion of an exclusive commercialization... ► Artikel lesen | |
| EISAI CO LTD ADR Aktie jetzt für 0€ handeln | |||||
| Di | GHIT Fund: Total Investment of Approx. USD 8.8 Million in Malaria, Tuberculosis, and NTD R&D Projects with Partners Including Mahidol University, Barcelona Institute for Global Health, and Eisai | 920 | PR Newswire | TOKYO, Feb. 3, 2026 /PRNewswire/ -- The Global Health Innovative Technology (GHIT) Fund announced today a total investment of approximately JPY 1.39 billion (USD 8.8 million1) in six R&D... ► Artikel lesen | |
| 27.01. | FDA to review Eisai's Leqembi Iqlik sBLA for Alzheimer's | 13 | Pharmaceutical Technology | ||
| 26.01. | BioArctic: Eisai submits Marketing Authorisation Variation to EMA for intravenous maintenance dosing every four weeks with Leqembi (lecanemab) | 407 | PR Newswire | STOCKHOLM, Sweden, Jan. 26, 2026 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai announced today that they have submitted a proposed Marketing Authorisation... ► Artikel lesen | |
| 26.01. | Eisai and Biogen's subcutaneous Leqembi given FDA Priority Review for early Alzheimer's | 7 | PMLiVE | ||
| 26.01. | BioArctic's Partner Eisai Receives Priority Review For Leqembi Iqlik Subcutaneous Autoinjector | 293 | AFX News | TOKYO (dpa-AFX) - BioArctic AB (BRCTF) announced on Monday that its partner Eisai (ESALY) has received Priority Review from the U.S. Food and Drug Administration (FDA) for the supplemental Biologics... ► Artikel lesen | |
| 26.01. | FDA grants priority review for Eisai's Alzheimer's treatment autoinjector | 8 | Investing.com | ||
| 26.01. | Eisai: FDA Accepts LEQEMBI(R) IQLIK (lecanemab-irmb) Supplemental Biologics License Application as a Subcutaneous Starting Dose for the Treatment of Early Alzheimer's Disease under Priority Review | 572 | JCN Newswire | If approved, LEQEMBI IQLIK would be the first and only anti-amyloid treatment to offer at-home injection options for initiation and maintenance dosing for this progressive, relentless disease FDA action... ► Artikel lesen | |
| 26.01. | FDA Grants Priority Review To Eisai And Biogen's SBLA For LEQEMBI Subcutaneous Autoinjector | 624 | AFX News | WESTON (dpa-AFX) - Eisai Co., Ltd. (ESALY, ESALF, 4523.T) and Biogen Inc. (BIIB) announced that the U.S. Food and Drug Administration has accepted for review Eisai's Supplemental Biologics License... ► Artikel lesen | |
| 26.01. | FDA accepts Eisai's autoinjector for Alzheimer's treatment under priority review | 5 | Investing.com | ||
| 26.01. | Eisai Inc.: FDA Accepts LEQEMBI IQLIKTM (lecanemab-irmb) Supplemental Biologics License Application as a Subcutaneous Starting Dose for the Treatment of Early Alzheimer's Disease under Priority Review | 1.541 | PR Newswire | If approved, LEQEMBI IQLIK would be the first and only anti-amyloid treatment to offer at-home injection options for initiation and maintenance dosing for this progressive... ► Artikel lesen | |
| 21.01. | Eisai Listed as a Global 100 Most Sustainable Corporation for the Tenth Time | 317 | JCN Newswire | Highest ranked global pharmaceutical companyTOKYO, Jan 21, 2026 - (JCN Newswire) - Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, "Eisai") announced today that it has been listed in the 2026... ► Artikel lesen | |
| 13.01. | Eisai and Nuvation Bio Enter into Exclusive Licensing Agreement for Taletrectinib in Europe and Additional Countries Outside the U.S., China and Japan | 665 | JCN Newswire | - Eisai will receive exclusive development, registration and commercialization rights for taletrectinib for the treatment of ROS1-positive non-small cell lung cancer in Europe, the Middle East, Canada... ► Artikel lesen | |
| 12.01. | Eisai sichert sich Lizenz für Lungenkrebs-Medikament Taletrectinib in Europe und Asien | 7 | Investing.com Deutsch | ||
| 07.01. | Newron Pharmaceuticals S.p.A.: EA Pharma, a subsidiary of Eisai, Announces the Initiation of its Phase III Clinical Trial with Evenamide, a Novel Treatment for Schizophrenia, in Japan | 804 | Business Wire | Newron Pharmaceuticals S.p.A. ("Newron") (SIX: NWRN, XETRA: NP5), a biopharmaceutical company focused on the development of novel therapies for patients with diseases of the central and peripheral... ► Artikel lesen |
1 2 3 4 5 Weiter >>
130 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 45,800 | +2,20 % | Lösung für Milliarden-Dollar-Problem: MustGrow Biologics validiert Geschäftsmodell - revolutionäre Meldung auch für Bayer und Corteva Agriscience | Raps ist für Kanada fast das, was Erdöl für Saudi-Arabien ist: Ein wirtschaftlicher Motor von gigantischem Ausmaß. Mit einem geschätzten Produktionswert von rund 14 Mrd. CAD im Jahr 2025 ist die gelb... ► Artikel lesen | |
| NOVO NORDISK | 40,310 | +0,01 % | Novo Nordisk Aktie: Aufgepasst, denn es wird spannend! Das Golden Cross rückt näher! | © Foto: 2026 Novo Nordisk A/SDie Novo Nordisk-Aktie steht vor einem entscheidenden charttechnischen Signal. Nach dem brutalen Absturz könnte sich das Blatt wenden. Der Kurs kämpft sich zurück, die wichtigen... ► Artikel lesen | |
| PFIZER | 23,015 | -0,07 % | Pfizer: Gute Zahlen, neue Daten - Kampfansage an Novo Nordisk und Co | Der Pharma-Gigant Pfizer hat am Dienstag die Zahlen für das abgelaufene vierte Quartal respektive Gesamtjahr 2025 veröffentlicht. Diese liegen über dem Markterwartungen. Darüber hinaus veröffentlichten... ► Artikel lesen | |
| NOVARTIS | 132,06 | +0,03 % | Novartis Pharma AG: Novartis erzielte im Jahr 2025 eine Umsatzsteigerung im hohen einstelligen Prozentbereich, eine Kerngewinnmarge von 40% sowie weitere Fortschritte in der Pipeline | Ad-hoc-Mitteilung gemäss Art. 53 KR
Geschäftsjahr
Der Nettoumsatz wuchs um +8% (kWk1, +8% USD), das operative Kernergebnis1 verbesserte sich um +14% (kWk, +12% USD)
Das Umsatzwachstum... ► Artikel lesen | |
| GILEAD SCIENCES | 128,98 | -0,05 % | Gilead wins favorable U.S. label update for Yescarta | ||
| AURORA CANNABIS | 2,925 | -0,85 % | Aurora Cannabis Inc.: Aurora Cannabis Announces Fiscal 2026 Third Quarter Results | NASDAQ | TSX: ACB
Expands YoY Total Net Revenue1 by 7% to $94.2 million, with Global Medical Cannabis Net Revenue1 Increasing by 12% to a Record $76.2 million
... ► Artikel lesen | |
| GSK | 25,300 | +1,12 % | GSK: Besser als erwartet | Der Pharmakonzern konnte ein unerwartet starkes Schlussquartal vorweisen und damit besser abschneiden als gedacht. Der Umsatz von GSK klettert im vergangenen Jahr um 4 % auf knapp 32,7 Mrd. Pfund, währungsbereinigt... ► Artikel lesen | |
| ABBVIE | 189,20 | +0,11 % | Aktien New York Ausblick: Keine Tech-Erholung - Eli Lilly steigen kräftig | NEW YORK (dpa-AFX) - Mit Blick auf den Technologiesektor dürften sich die Investoren an den US-Börsen am Mittwoch zurückhalten. Der Nasdaq 100 Index, der am Vortag deutlich gefallen war, dürfte sich... ► Artikel lesen | |
| CANOPY GROWTH | 0,935 | -0,95 % | Canopy Growth: Treffer ohne Gewinn - Trading-Tipp wird zurückgezogen | ||
| ROCHE | 391,10 | -0,29 % | DZ BANK stuft ROCHE HOLDINGS AG auf 'Kaufen' | FRANKFURT (dpa-AFX Analyser) - Die DZ Bank hat den fairen Aktienwert für Roche von 324 auf 395 Franken angehoben und die Einstufung auf "Kaufen" belassen. "Die Pharma-Sparte hat den großen, nahezu parallelen... ► Artikel lesen | |
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 894,80 | -0,01 % | Novo Nordisk-Aktie stürzt ab! Was macht Eli Lilly? Hochspannung vor Quartalszahlen des US-Konzerns | Der dänische Pharmariese Novo Nordisk steht unter massivem Druck: Umsatzwarnung, Kurssturz und scharfer internationaler Wettbewerb setzen dem Konzern zu. Anleger blicken nun gespannt auf die Zahlen... ► Artikel lesen | |
| ASTRAZENECA | 160,70 | 0,00 % | Erfolg für AstraZeneca | Der Pharmakonzern hat gestern gemeldet, dass sein Medikament Imfinzi in Kombination mit einer Chemotherapie für die Behandlung erwachsener Patienten mit Magen- sowie gastroösophagealen Übergangstumoren... ► Artikel lesen | |
| TILRAY BRANDS | 6,450 | +0,62 % | Tilray Brands, Inc.: CC Pharma von Tilray als eines der 100 innovativsten Unternehmen in Deutschland ausgezeichnet | Die Auszeichnung würdigt CC Pharma als innovativen Marktführer und vertrauenswürdigen Partner auf dem europäischen PharmamarktDENSBORN, Deutschland, Feb. 04, 2026 (GLOBE NEWSWIRE) -- Tilray Pharma,... ► Artikel lesen | |
| TEVA | 29,300 | -0,34 % | Teva Canada Announces Approval of Expanded Indication of [Pr]AJOVY (fremanezumab solution for subcutaneous injection), the First Anti-CGRP Preventive Treatment for Pediatric Episodic Migraine |
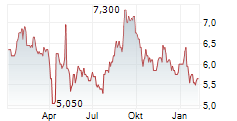
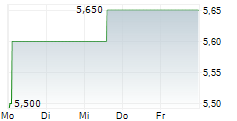
EISAI CO LTD ADR gehört der Branche Pharma an. Mit einer aktuellen Kursveränderung von 0,000 (0,00 %) liegt der Kurs bei 5,650 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu EISAI CO LTD ADR finden Sie auf Wallstreet Online.