| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 0,081 | 0,087 | 13:05 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 29.01. | GenSight Biologics S.A.: GenSight Biologics Announces the Results of its Extraordinary General Meeting of January 28, 2026 | 802 | Business Wire | Regulatory News:
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal... ► Artikel lesen | |
| 08.01. | GenSight Biologics Reports Cash Position as of December 31, 2025, and Provides Business Updates | 318 | Business Wire | Cash position amounted to €2.4 million as of December 31, 2025
Successful closing of fundraising worth nearly €2.9 million on January 7, 2026
Payments for early access treatments... ► Artikel lesen | |
| 07.01. | GenSight Biologics Announces Closing of Fundraising Worth Nearly EUR 2.9 Million | 296 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics (the "Company") (Euronext: SIGHT... ► Artikel lesen | |
| GENSIGHT BIOLOGICS Aktie jetzt für 0€ handeln | |||||
| 29.12.25 | GenSight Biologics Announces Successful Fundraising Amounting to Nearly €2.9 Million | 359 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics ("GenSight" or the "Company") (Euronext:... ► Artikel lesen | |
| 22.12.25 | GenSight Biologics S.A.: GenSight Biologics Announces Regulatory Authorization for Early Access Treatment with GS010/LUMEVOQ in Israel | 415 | Business Wire | Israel's Ministry of Health authorizes individual patients early access treatment with GS010/LUMEVOQ, a candidate gene therapy for the treatment of ND4-LHON.
Bilateral injection expected... ► Artikel lesen | |
| 22.12.25 | GenSight Biologics S.A.: GenSight Biologics Announces the Granting of Compassionate Use Authorization (CUA/AAC) for GS010/LUMEVOQ in France | 432 | Business Wire | French medicines agency ANSM has granted authorization for named patient early access program for GS010/LUMEVOQ, a gene therapy indicated for the treatment of ND4-LHON.
Regulatory News:
GenSight... ► Artikel lesen | |
| 02.12.25 | GenSight Biologics Announces Regulatory Approval for GS010/LUMEVOQ REVISE Dose-Ranging Study in France | 338 | Business Wire | French medicines agency ANSM and Ethics Committee authorize the dose-ranging study investigating two doses of candidate product for the treatment of ND4-LHON.
Approval of the study protocol... ► Artikel lesen | |
| 13.11.25 | GenSight Biologics Announces Closing of Fundraising Worth Nearly EUR 2 Million | 415 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics (the "Company") (Euronext: SIGHT... ► Artikel lesen | |
| 10.11.25 | GenSight Biologics S.A.: GenSight Biologics Announces Successful Fundraising Amounting to Nearly €2 Million | 279 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics ("GenSight" or the "Company") (Euronext:... ► Artikel lesen | |
| 30.10.25 | GenSight Biologics Announces Regulatory Authorizations for Individual Patient Expanded Access Treatment with GS010/LUMEVOQ in the US | 487 | Business Wire | FDA authorization and Institutional Review Board (IRB) approval followed by QP release for expanded access treatment of one patient in the US
Treatment scheduled in November 2025 at the... ► Artikel lesen | |
| 07.10.25 | GenSight Biologics Reports Cash Position as of September 30, 2025 | 355 | Business Wire | Cash position amounted to €0.6 million as of September 30, 2025
Successful closing of fundraising worth nearly EUR3.7 million on October 1, 2025
Cash position amounted to €3.6 million... ► Artikel lesen | |
| 01.10.25 | GenSight Biologics S.A.: GenSight Biologics Announces Closing of Fundraising Worth Nearly EUR 3.7 Million | 510 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics (the "Company") (Euronext: SIGHT... ► Artikel lesen | |
| 29.09.25 | GenSight Biologics Reports Interim Financial Results for the First Half of 2025 | 486 | Business Wire | Optimized cash management results in 8.3% reduction in operating cash outflow compared to the same period in 2024
Cash runway extended to late Q4 2025 following post-closing financing
... ► Artikel lesen | |
| 26.09.25 | GenSight Biologics S.A.: GenSight Biologics Announces Successful Fundraising Amounting to Nearly EUR 3.7 Million | 346 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics (the "Company") (Euronext: SIGHT... ► Artikel lesen | |
| 28.08.25 | GenSight Biologics S.A.: GenSight Biologics Postpones Release of 2025 Half-Year Financial Results | 462 | Business Wire | Regulatory News:
GenSight Biologics ("GenSight Biologics" or the "Company") (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing... ► Artikel lesen | |
| 22.07.25 | GenSight Biologics Announces the Closing of Approximately €500,000 of Additional Financing | 447 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics (the "Company") (Euronext: SIGHT... ► Artikel lesen | |
| 17.07.25 | GenSight Biologics Announces Publication on Predictors of Final Visual Outcome in Patients Treated with LUMEVOQ Gene Therapy | 406 | Business Wire | Better baseline visual acuity and thicker baseline optical coherence tomography (OCT) parameters predict better final visual outcome
Patients treated during dynamic phase achieve better... ► Artikel lesen | |
| 17.07.25 | GenSight Biologics Announces an Additional Financing Amounting to Approximately EUR 500,000 from Existing Shareholder | 504 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics (the "Company") (Euronext: SIGHT... ► Artikel lesen | |
| 08.07.25 | GenSight Biologics Reports Cash Position as of June 30, 2025 | 481 | Business Wire | Operations funded until early October 2025 as a result of disciplined spending controls
Agreement with the ANSM to consider opening the Named Early Access Program (AAC) for LUMEVOQ in France... ► Artikel lesen | |
| 03.07.25 | GenSight Biologics Announces the Closing of the Company's c. €4 Million Private Placement | 539 | Business Wire | Regulatory News:
NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, CANADA, AUSTRALIA, JAPAN AND SOUTH AFRICA
GenSight Biologics (the "Company") (Euronext: SIGHT... ► Artikel lesen |
1 2 Weiter >>
32 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 90,40 | +0,17 % | Startschuss! BioTech-Sektor profitiert von Rotation! Augen auf bei Evotec, Bayer, Vidac Pharma and BioNTech | Mit positiver Grundstimmung hat die Börse das Jahr 2026 eingeläutet. Dass Renditechancen nicht allein im Technologiesektor liegen, bewies zuletzt der Minen- und Rohstoffbereich, wo etliche Titel Kursverdopplungen... ► Artikel lesen | |
| EVOTEC | 6,148 | +0,79 % | SDAX: Evotec-Aktie schießt zweistellig nach oben | Eine Kaufempfehlung von Berenberg entfacht neuen Optimismus bei Evotec und katapultiert die Biotech-Aktie an die Indexspitze. Die Aktie der Evotec hat am Dienstagmittag ein Kursfeuerwerk gezündet und... ► Artikel lesen | |
| BB BIOTECH | 50,60 | -0,78 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| MEDIGENE | 0,042 | -8,26 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 43,170 | -0,70 % | Ionos, Kontron, Lanxess, Qiagen, Redcare Pharmacy, Siltronic, TeamViewer: Neue Aktien-Positionen der Short-Seller | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| MODERNA | 34,650 | -0,07 % | Arbutus jumps on ruling in patent dispute with Moderna | ||
| PAION | 0,200 | +8,11 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WIRD GEPRUEFT | Fuer folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wird ein Mistrade-Antrag geprueft:ISIN Datum Zeit Volumen Preis Fair Value (AS)DE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 3,986 | +0,61 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| AMGEN | 325,15 | +0,06 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| EPIGENOMICS | 0,870 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 |
DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 6,910 | -0,80 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| STRYKER | 302,10 | -0,33 % | Stryker Corporation: Stryker declares an $0.88 per share quarterly dividend | Portage, Michigan, Feb. 04, 2026 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) announced that its Board of Directors has declared a quarterly dividend of $0.88 per share payable April 30, 2026, to shareholders... ► Artikel lesen | |
| BIOGEN | 164,25 | +3,79 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,420 | -1,22 % | PTA-news: Biofrontera AG: Biofrontera berichtet über die Ergebnisse der ersten neun Monate des Jahres 2025 | DJ PTA-News: Biofrontera AG: BIOFRONTERA BERICHTET ÜBER DIE ERGEBNISSE DER ERSTEN NEUN MONATE DES JAHRES 2025
Unternehmensmitteilung für den Kapitalmarkt
Biofrontera AG: BIOFRONTERA... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,910 | 0,00 % | HEIDELBERG PHARMA AG zeigt klare Stärke im Trend |
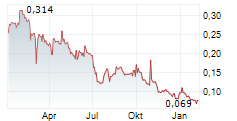
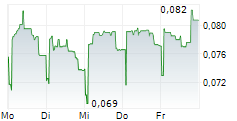
GENSIGHT BIOLOGICS SA gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von 0,000 (0,00 %) liegt der Kurs bei 0,081 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu GENSIGHT BIOLOGICS SA finden Sie auf Wallstreet Online.