- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 25.03. | Guard Therapeutics International AB: Two-thirds of patients enrolled in Guard Therapeutics' Phase 2b POINTER study | 127 | GlobeNewswire (Europe) | Guard Therapeutics announced today that it has reached another key milestone in its ongoing clinical development program. Two-thirds of the approximately 160 planned patients have now been enrolled... ► Artikel lesen | |
| 14.03. | XFRA CAPITAL ADJUSTMENT INFORMATION - 14.03.2025 | 657 | Xetra Newsboard | Das Instrument OBD SE0000514705 OBDUCAT AB B SK 8 EQUITY wird cum Kapitalmassnahme gehandelt am 14.03.2025 und ex Kapitalmassnahme am 17.03.2025 The instrument OBD SE0000514705 OBDUCAT AB B SK 8 EQUITY... ► Artikel lesen | |
| 10.03. | Guard Therapeutics International AB: The Board of Directors of Guard Therapeutics has resolved on a previously announced rights issue of approximately SEK 150 million | 95 | GlobeNewswire (Europe) | THIS PRESS RELEASE MAY NOT BE MADE PUBLIC, PUBLISHED OR DISTRIBUTED, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES OF AMERICA, AUSTRALIA, BELARUS, HONG KONG, JAPAN, CANADA, NEW ZEALAND, RUSSIA... ► Artikel lesen | |
| 27.02. | Guard Therapeutics International AB: Guard Therapeutics announces positive outcome from first independent safety data review in Phase 2b POINTER study | 82 | GlobeNewswire (Europe) | Guard Therapeutics announced today that an independent Data Safety Monitoring Committee (DSMC) has completed its first planned review of safety data and has recommended that the Phase 2b clinical study... ► Artikel lesen | |
| 04.09.24 | Nasdaq Stockholm AB: Listing of subscription rights and paid subscription shares of Guard Therapeutics International AB | 388 | GlobeNewswire | With effect from September 05, 2024, the subscription rights in Guard
Therapeutics International AB will be traded on First North Growth Market.
Trading will continue up until and including September... ► Artikel lesen | |
| GUARD THERAPEUTICS INTERNATIONAL Aktie jetzt für 0€ handeln | |||||
| 02.09.24 | XFRA CAPITAL ADJUSTMENT INFORMATION - 02.09.2024 | 547 | Xetra Newsboard | Das Instrument MSPA MA0000011488 ITISSALAT AL-MA. INH.DH 6 EQUITY wird cum Kapitalmassnahme gehandelt am 02.09.2024 und ex Kapitalmassnahme am 03.09.2024 The instrument MSPA MA0000011488 ITISSALAT AL-MA.... ► Artikel lesen | |
| 29.08.24 | Guard Therapeutics International AB: First patient dosed in Guard Therapeutics' phase 2b study POINTER | 215 | GlobeNewswire (Europe) | Guard Therapeutics today announced that the first patient has been dosed in the phase 2b clinical study POINTER with the drug candidate RMC-035 as a kidney-protective treatment in open-heart surgery.
"It... ► Artikel lesen | |
| 27.08.24 | Guard Therapeutics International AB: Guard Therapeutics carries out announced rights issue (repair issue) | 185 | GlobeNewswire (Europe) | THIS PRESS RELEASE MAY NOT BE MADE PUBLIC, DISTRIBUTED OR PUBLISHED, DIRECTLY OR INDIRECTLY, IN OR INTO THE UNITED STATES OF AMERICA, AUSTRALIA, BELARUS, CANADA, HONG KONG, JAPAN, NEW ZEALAND, RUSSIA... ► Artikel lesen |
8 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| NOVONESIS | 55,72 | +0,76 % | Novonesis (Novozymes A/S): Transactions under Novonesis' share buyback program | ||
| GENMAB | 174,40 | +0,29 % | Genmab A/S: Genmab Announces Net Sales of DARZALEX (daratumumab) for First Quarter of 2025 | Company Announcement Net sales of DARZALEX® in the first quarter of 2025 totaled USD 3,237 millionGenmab receives royalties on worldwide net sales from Johnson & Johnson (J&J, legal entity Janssen... ► Artikel lesen | |
| ARBUTUS BIOPHARMA | 2,618 | -5,62 % | Arbutus Biopharma Corporation: Arbutus Reports Fourth Quarter and Year End 2024 Financial Results and Provides Corporate Update | Claim construction hearing for Pfizer/BioNTech mRNA-LNP vaccine litigation occurred in December 2024; outcome pending Jury trial in Moderna U.S. mRNA-LNP vaccine litigation scheduled for September... ► Artikel lesen | |
| MOLECULIN BIOTECH | 0,735 | -100,00 % | Moleculin Biotech, Inc. - 10-K/A, Annual Report | ||
| VERICEL | 36,200 | +2,26 % | Vericel Corp - 8-K, Current Report | ||
| SENSEI BIOTHERAPEUTICS | 0,337 | -5,07 % | Sensei Biotherapeutics Reports Full Year 2024 Financial Results and Update on Clinical Progress | - Preliminary efficacy data from Phase 1/2 dose expansion cohort show durable responses and tumor shrinkage in a PD-(L)1 resistant "hot" tumor patient population - - Solnerstotug continues to demonstrate... ► Artikel lesen | |
| BIOXCEL THERAPEUTICS | 1,365 | -100,00 % | BioXcel Therapeutics Strengthens Cash Position to Advance SERENITY At-Home Pivotal Phase 3 Safety Trial for Acute Treatment of Agitation Associated with Bipolar Disorders or Schizophrenia | Topline data expected in second half of 2025 to support potential sNDA submission for label expansion of IGALMI® in the home setting Company has $35M of cash following closing of recent equity financing... ► Artikel lesen | |
| MUSTGROW BIOLOGICS | 0,638 | -6,18 % | MustGrow Biologics Corp.: MustGrow Adds New Biostimulants and Inoculants to Canadian Product Portfolio | Saskatoon, Saskatchewan--(Newsfile Corp. - April 1, 2025) - MustGrow Biologics Corp. (TSXV: MGRO) (OTCQB: MGROF) (FSE: 0C0) (the "Company" or "MustGrow"), a leading provider of biological and regenerative... ► Artikel lesen | |
| REGENXBIO | 5,250 | 0,00 % | Regenxbio Aktie: Überschaubare Aussichten | Regenxbio verzeichnet aktuell einen Kursrückgang von 3,45 Prozent auf 7,00 Euro, wobei die Aktie in den letzten sieben Tagen sogar über 13 Prozent an Wert eingebüßt hat. Das Unternehmen hat kürzlich... ► Artikel lesen | |
| AKEBIA | 1,880 | +3,98 % | Akebia Therapeutics, Inc.: Akebia Therapeutics Announces Positive Opinion of European Medicines Agency for XOANACYL, an Oral Therapy for Chronic Kidney Disease Licensed to Averoa | CAMBRIDGE, Mass., April 03, 2025 (GLOBE NEWSWIRE) -- Akebia Therapeutics®, Inc. (Nasdaq: AKBA), a biopharmaceutical company with the purpose to better the lives of people impacted by kidney disease... ► Artikel lesen | |
| FENNEC PHARMACEUTICALS | 4,560 | 0,00 % | Fennec Pharmaceuticals Inc.: Fennec Pharmaceuticals Reports Fourth Quarter and Full-Year 2024 Financial Results and Provides Business Update | ~ Achieved Full-Year PEDMARK® Net Product Sales of $29.6 Million, Up 40% Year-Over-Year, and Generated PEDMARK® Q4 2024 Net Product Sales of $7.9 Million ~ ~ Delivered Q4 2024 EBITDA Loss of $0.6... ► Artikel lesen | |
| ZEVRA THERAPEUTICS | 6,250 | -0,79 % | Zevra Therapeutics: Zevra Announces Publication of MIPLYFFA Mechanism of Action Manuscript in Molecular Genetics and Metabolism | ||
| REVANCE THERAPEUTICS | 3,420 | 0,00 % | XFRA DELETION OF INSTRUMENTS FROM BOERSE FRANKFURT - 07.02.2025 | The following instruments on Boerse Frankfurt do have their last trading day on 07.02.2025Die folgenden Instrumente in Boerse Frankfurt haben ihren letzten Handelstag am 07.02.2025ISIN NameCA09226M1005 BLACK... ► Artikel lesen | |
| CORVUS PHARMACEUTICALS | 2,880 | +1,23 % | Corvus Pharmaceuticals, Inc.: Corvus Pharmaceuticals Provides Business Update and Reports Fourth Quarter and Full Year 2024 Financial Results | Soquelitinib Atopic Dermatitis Phase 1 Clinical Trial Data From Cohorts 1-3 anticipated to be Presented in May Initial Results From First Two Cohorts Supports Safety and Efficacy with Oral Agent and... ► Artikel lesen | |
| CARTESIAN THERAPEUTICS | 10,000 | -6,54 % | Cartesian Therapeutics, Inc.: Cartesian Therapeutics' Descartes-08 Observed to Provide Deep and Sustained Benefits Through Month 12 After a Single Course of Therapy in Phase 2b Myasthenia Gravis Trial | After a single course of therapy, Descartes-08-treated participants were observed to sustain deep responses through long-term follow-up, with an average 4.8-point reduction in MG-ADL at Month 12 Deepest... ► Artikel lesen |
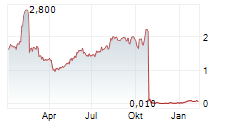
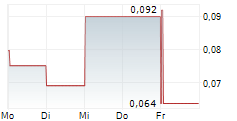
Das Unternehmen GUARD THERAPEUTICS INTERNATIONAL AB kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell 0,00 % bei einem Aktienkurs von 1,020 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und .
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu GUARD THERAPEUTICS INTERNATIONAL AB finden Sie auf Wallstreet Online.