- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mo | HUTCHMED Ltd - 6-K, Report of foreign issuer | - | SEC Filings | ||
| Mo | Hutchmed-Aktionäre genehmigen wichtigen Aktienverkauf | 2 | Investing.com Deutsch | ||
| Mo | Hutchmed shareholders approve key equity sale | 1 | Investing.com | ||
| HUTCHMED CHINA LIMITED ADR Aktie jetzt für 0€ handeln | |||||
| Mo | HUTCHMED (00013): EXTRAORDINARY GENERAL MEETING HELD ON MARCH 31, 2025 - POLL RESULTS | - | HKEx | ||
| Mo | Hutchmed China Ltd - Extraordinary General Meeting - Poll Results | - | RNS | ||
| 24.03. | CMBI Cuts HUTCHMED TP to $34.03, Rating Buy | 3 | AASTOCKS | ||
| 21.03. | China conditionally approves Hutchmed's Tazverik for follicular lymphoma | 2 | Pharmaceutical Technology | ||
| 21.03. | HUTCHMED Announces Regulatory Approval For TAZVERIK In China | 275 | AFX News | BEIJING (dpa-AFX) - HUTCHMED China (HCM) announced that the New Drug Application for TAZVERIK has been granted conditional approval in China for the treatment of adult patients with relapsed... ► Artikel lesen | |
| 21.03. | HUTCHMED Ltd - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 21.03. | HUTCHMED gains NMPA nod for lymphoma drug TAZVERIK | 2 | Investing.com | ||
| 21.03. | HUTCHMED erhält NMPA-Zulassung für Lymphom-Medikament TAZVERIK | 2 | Investing.com Deutsch | ||
| 21.03. | HUTCHMED (China) Limited: HUTCHMED Announces NMPA Conditional Approval for TAZVERIK (tazemetostat) for the Treatment of Relapsed or Refractory Follicular Lymphoma | 108 | GlobeNewswire (Europe) | - First and only EZH2 inhibitor approved by the NMPA - - HUTCHMED's fourth product, and its first approval in hematological malignancies - HONG KONG and SHANGHAI and FLORHAM PARK, N.J., March 21,... ► Artikel lesen | |
| 21.03. | Hutchmed China Ltd - NMPA Conditional Approval for TAZVERIK® | - | RNS | ||
| 21.03. | HUTCHMED (00013): VOLUNTARY ANNOUNCEMENT - HUTCHMED ANNOUNCES NMPA CONDITIONAL APPROVAL FOR TAZVERIK (TAZEMETOSTAT) FOR THE TREATMENT OF RELAPSED OR REFRACTORY ... | - | HKEx | ||
| 21.03. | Daiwa Cuts HUTCHMED TP to $33, Rating Buy Kept | 2 | AASTOCKS | ||
| 20.03. | Hutchmed announces board reshuffle as directors retire | 1 | Investing.com | ||
| 20.03. | Hutchmed kündigt Vorstandsumstrukturierung nach Rücktritt von Direktoren an | 1 | Investing.com Deutsch | ||
| 20.03. | HUTCHMED (China) Limited: Intended Retirement of Independent Non-executive Directors and changes of composition of board committees | 5 | GlobeNewswire (USA) | ||
| 20.03. | HUTCHMED (00013): INTENDED RETIREMENT OF INDEPENDENT NON-EXECUTIVE DIRECTORS AND CHANGES OF COMPOSITION OF BOARD COMMITTEES | 1 | HKEx | ||
| 20.03. | Hutchmed China Ltd - Retirement of Independent Non-executive Directors | - | RNS |
Seite:
1 2 3 4 5 Weiter >>
185 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 20,095 | -5,50 % | wO Börsenlounge - die Woche: Von Bayers Murmeltier, Teslas Querelen und der Situation bei Mutares/Steyr | Bayer verliert mal wieder eine Klage in den USA, bei Tesla gibt es auffällig viele Insiderverkäufe und die Achterbahnfahrt bei Mutares/Steyer könnte ein gerichtliches Nachspiel haben. Die Woche mit... ► Artikel lesen | |
| NOVO NORDISK | 57,62 | +0,19 % | AKTIONÄR-Depotwert Gubra: Im Sog von Novo Nordisk - Daten voraus | Die Aktie des dänischen Biopharma-Riesen Novo Nordisk fällt und fällt. Der Abverkauf geht auch an kleineren Playern im Adipositas-Bereich nicht spurlos vorbei. So auch beim AKTIONÄR-Depotwert Gubra:... ► Artikel lesen | |
| PFIZER | 21,000 | -0,02 % | JPMORGAN stuft PFIZER INC auf 'Neutral' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für Pfizer vor Zahlen zum ersten Quartal auf "Neutral" mit einem Kursziel von 30 US-Dollar belassen. Analyst Chris Schott sieht... ► Artikel lesen | |
| NOVARTIS | 96,77 | -5,26 % | Novartis: Enorm wichtiger Meilenstein - Marktpotenzial fast verdreifacht | Der Pharma-Riese Novartis konnte am vergangenen Freitag eine enorm wichtige Zulassung verbuchen. Das radiopharmazeutische Medikament Pluvicto darf in den USA fortan in einer weiteren Indikation vertrieben... ► Artikel lesen | |
| MERCK KGAA | 117,75 | -3,64 % | DZ BANK stuft MERCK KGAA auf 'Kaufen' | FRANKFURT (dpa-AFX Analyser) - Die DZ Bank hat den fairen Wert für die Aktie von Merck KGaA von 201 auf 178 Euro gesenkt, jedoch die Einstufung auf "Kaufen" belassen. Analyst Peter Spengler begründete... ► Artikel lesen | |
| GILEAD SCIENCES | 98,00 | -0,05 % | Gilead Sciences: The Centrifuge Sessions: Driving Innovation and Investment in Cancer Research | NORTHAMPTON, MA / ACCESS Newswire / April 3, 2025 / We're focused on driving innovation in virology, oncology and immunology - and that includes investing in world-class science to change the way cancer... ► Artikel lesen | |
| AURORA CANNABIS | 3,656 | -0,11 % | Aurora Cannabis Aktie: Ein neuer Lichtblick! | Der kanadische Cannabisproduzent positioniert sich durch internationale Markterschließung, technologische Innovation und solide Finanzlage für langfristiges Wachstum. Aurora Cannabis zieht weiterhin... ► Artikel lesen | |
| SANOFI | 96,07 | -3,64 % | Ihre wichtigsten Termine: DHL, Volvo, Auto1 Group, Sanofi und VDMA legen Spannende Updates vor! | © Foto: picture alliance/AA/Guillaume Payen - picture alliance Gut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen... ► Artikel lesen | |
| GSK | 16,820 | -3,42 % | GSK Aktie: Entwicklung spiegelt Erwartungen | Der Pharmakonzern GSK navigiert durch Zantac-Klagen und entwickelt parallel neue Gesundheitslösungen, während die Finanzprognose bis 2031 optimistisch angehoben wurde. GSK steht derzeit vor komplexen... ► Artikel lesen | |
| ABBVIE | 170,22 | -0,32 % | AbbVie Aktie: Potenzial voll ausgeschöpft! | Der Pharmakonzern veröffentlicht seine Finanzdaten für Q1/2025 am 25. April und baut durch strategische Investitionen seine Position im wachsenden ADC-Markt aus. AbbVie wird seine Finanzergebnisse für... ► Artikel lesen | |
| CANOPY GROWTH | 0,845 | -0,24 % | Schock-News für Canopy Growth Anleger vor dem Wochenende: So müssen Sie heute noch auf diese Sondermeldung reagieren! | ||
| ROCHE | 275,55 | -5,60 % | INTERVIEW/Roche bei Krebsdiagnostik auf Next Generation Sequencing | DJ INTERVIEW/Roche bei Krebsdiagnostik auf Next Generation Sequencing
Von Helena Smolak
DOW JONES--Der Schweizer Pharmariese Roche Holding will sich angesichts der wachsenden Nachfrage nach... ► Artikel lesen | |
| STADA ARZNEIMITTEL | - | - | Laufende Fusionskontrollverfahren: Bain Capital Investors, LLC, Boston, MA, USA (u.a. Stada AG); mittelbarer Erwerb aller Anteile an und alleiniger Kontrolle über Mitsubishi Tanabe Pharma Corporation, Osaka, Japan | Datum der Anmeldung:25.03.2025Aktenzeichen:B3-43/25Unternehmen:Bain Capital Investors, LLC, Boston, MA, USA (u.a. Stada AG); mittelbarer Erwerb aller Anteile an und alleiniger Kontrolle über Mitsubishi... ► Artikel lesen | |
| MERCK & CO | 74,50 | 0,00 % | Merck Aktie: Keine neuen Risiken bekannt | News von Trading-Treff.de Merck & Co. hat kürzlich bedeutende Initiativen ergriffen, um seine Marktposition zu verbessern und bevorstehenden Patentabläufen entgegenzuwirken. Der Pharmakonzern schloss... ► Artikel lesen | |
| ELI LILLY | 683,30 | -5,37 % | Moderna im Sturzflug, auch BioNTech bekommt es mit Gegenwind zu tun, Rückschlag für Eli Lilly in Europa und Novo Nordisk bleibt trotz positiver Studiendaten ebenfalls im roten Bereich | Sollte es das Ziel der Trump-Administration sein, Aktienkurse möglichst spektakulär crashen zu lassen, so ist sie dabei recht erfolgreich. Schwere Verunsicherung herrschte jüngst aufgrund von neuen... ► Artikel lesen |
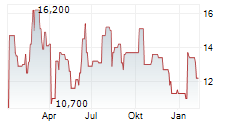
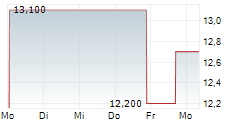
HUTCHMED CHINA LIMITED ADR gehört der Branche Pharma an. Mit einer aktuellen Kursveränderung von +2,100 (+15,91 %) liegt der Kurs bei 15,300 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und .
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu HUTCHMED CHINA LIMITED ADR finden Sie auf Wallstreet Online.