| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 2,420 | 2,440 | 17.04. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 07.04. | HUTCHMED Ltd - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 07.04. | Hutchmed plant Hauptversammlung 2025 für den 13. Mai | 1 | Investing.com Deutsch | ||
| 07.04. | HUTCHMED (00013): NOTIFICATION LETTER TO NON-REGISTERED SHAREHOLDERS AND REPLY FORM: (1) ANNUAL GENERAL MEETING ("AGM"); AND (2) NOTICE OF PUBLICATION ... | 1 | HKEx | ||
| 07.04. | HUTCHMED (00013): NOTIFICATION LETTER TO REGISTERED HOLDERS AND REPLY FORM: (1) ANNUAL GENERAL MEETING ("AGM"); AND (2) NOTICE OF PUBLICATION OF THE 2024 ... | 2 | HKEx | ||
| 07.04. | Hutchmed schedules 2025 AGM for May 13 | 2 | Investing.com | ||
| 07.04. | HUTCHMED (00013): 2024 ANNUAL REPORT AND NOTICE OF ANNUAL GENERAL MEETING | - | HKEx | ||
| HUTCHMED Aktie jetzt für 0€ handeln | |||||
| 07.04. | HUTCHMED (00013): FORM OF PROXY FOR USE AT THE ANNUAL GENERAL MEETING | - | HKEx | ||
| 07.04. | HUTCHMED (00013): NOTICE OF ANNUAL GENERAL MEETING | - | HKEx | ||
| 07.04. | HUTCHMED (00013): NOTICE OF ANNUAL GENERAL MEETING; PROPOSALS FOR RE-ELECTION OF DIRECTORS AND GENERAL MANDATES TO ISSUE NEW SHARES AND REPURCHASE SHARES | - | HKEx | ||
| 07.04. | HUTCHMED (00013): 2024 SUSTAINABILITY REPORT | 2 | HKEx | ||
| 07.04. | Hutchmed China Ltd - 2024 Annual Report and Notice of AGM | - | RNS | ||
| 07.04. | HUTCHMED (00013): 2024 ANNUAL REPORT | - | HKEx | ||
| 31.03. | HUTCHMED Ltd - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 31.03. | Hutchmed-Aktionäre genehmigen wichtigen Aktienverkauf | 2 | Investing.com Deutsch | ||
| 31.03. | Hutchmed shareholders approve key equity sale | 1 | Investing.com | ||
| 31.03. | HUTCHMED (00013): EXTRAORDINARY GENERAL MEETING HELD ON MARCH 31, 2025 - POLL RESULTS | - | HKEx | ||
| 31.03. | Hutchmed China Ltd - Extraordinary General Meeting - Poll Results | - | RNS | ||
| 24.03. | CMBI Cuts HUTCHMED TP to $34.03, Rating Buy | 3 | AASTOCKS | ||
| 21.03. | China conditionally approves Hutchmed's Tazverik for follicular lymphoma | 2 | Pharmaceutical Technology | ||
| 21.03. | HUTCHMED Announces Regulatory Approval For TAZVERIK In China | 304 | AFX News | BEIJING (dpa-AFX) - HUTCHMED China (HCM) announced that the New Drug Application for TAZVERIK has been granted conditional approval in China for the treatment of adult patients with relapsed... ► Artikel lesen |
Seite:
1 2 3 4 5 Weiter >>
192 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 21,030 | +0,36 % | Bayer-Aktie: Der Bewertungstrick! | News von Trading-Treff.de Bayer hat am Mittwoch mit dem Gewinn von 0,05 % die Börsensitzung recht bescheiden eröffnet. Der Kurs bleibt bei über 20 Euro und nahm nun 20,84 Euro ein. Dennoch ist und verharrt... ► Artikel lesen | |
| NOVO NORDISK | 51,88 | -8,68 % | Novo Nordisk: Überraschende Verkaufsempfehlung | Zur Wochenmitte befindet sich die Aktie von Novo Nordisk erneut auf dem absteigenden Ast. Zur Stunde verliert das Papier gut ein Prozent an Wert. Auf die Stimmung drückt eine Verkaufsempfehlung. Die... ► Artikel lesen | |
| PFIZER | 19,478 | +0,02 % | Blockbuster oder Übernahmefieber? Warum Novo Nordisk, Defence Therapeutics und Pfizer jetzt alle Blicke auf sich ziehen | Die Biotech- und Pharmabranche gleicht einem Schachbrett der Milliarden! Ein einziger Zug - ob Übernahme oder Zulassung - kann Aktienkurse in Stunden verdoppeln oder ganze Märkte neu ordnen. Während... ► Artikel lesen | |
| GILEAD SCIENCES | 91,99 | +0,08 % | Gilead Sciences: Advancing Oncology Care Through Gilead's Research Scholars Program | NORTHAMPTON, MA / ACCESS Newswire / April 15, 2025 / As a medical oncologist who has practiced in three different countries, Dr. Maria Alice Franzoi is well acquainted with various healthcare systems... ► Artikel lesen | |
| AURORA CANNABIS | 3,860 | -1,28 % | Aurora Cannabis Inc (3): Aurora Cannabis offers inhalers to U.K. patients | ||
| GSK | 15,710 | -0,32 % | GSK erhält in den USA Empfehlungen für Meningitis- und RSV-Impfstoffe | DJ GSK erhält in den USA Empfehlungen für Meningitis- und RSV-Impfstoffe
Von Cristina Gallardo
DOW JONES--Der britische Pharmakonzern GSK hat von einem Beratungsgremium des US-Gesundheitsministeriums... ► Artikel lesen | |
| ABBVIE | 152,20 | 0,00 % | AbbVie Aktie: Nichts wird wie früher! | Der Pharmakonzern verzeichnet erhebliche Kursverluste von 33% unter Jahreshöchststand, während Experten weiterhin optimistisch bleiben und ein Kursziel von 195 Euro anvisieren. Der Pharmakonzern AbbVie... ► Artikel lesen | |
| STADA ARZNEIMITTEL | - | - | MIDDAY BRIEFING - Unternehmen und Märkte: CONTINENTAL, DAIMLER TRUCK, PORSCHE, HUGO BOSS, AUMANN, BAYWA, STADA, BYD, OMV, STELLANTIS | DJ MIDDAY BRIEFING - Unternehmen und Märkte
Der Markt-Überblick am Mittag, zusammengestellt von Dow Jones Newswires:
+++++ AKTIENMÄRKTE (13:18) +++++
zuletzt +/-... ► Artikel lesen | |
| ASTRAZENECA | 118,75 | -0,92 % | Astrazeneca Aktie: Details zum strategischen Plan | Trotz aktueller Schwächephase halten Analysten an Kaufempfehlungen fest - Dividendenankündigung und Quartalszahlen im Fokus. Die AstraZeneca-Aktie zeigt sich diese Woche widersprüchlich: Während Aktionäre... ► Artikel lesen | |
| TILRAY BRANDS | 0,400 | +0,78 % | Tilray Brands, Inc.: Tilray Brands Announces Proposed Reverse Stock Split and Corresponding Special Meeting of Stockholders | NEW YORK and LEAMINGTON, Ontario, April 17, 2025 (GLOBE NEWSWIRE) -- Tilray Brands, Inc. ("Tilray" or "Company") (Nasdaq: TLRY; TSX: TLRY), a global lifestyle and consumer packaged goods company at... ► Artikel lesen | |
| BRISTOL-MYERS SQUIBB | 43,665 | +0,88 % | Bristol-Myers Squibb Aktie: Ringen um Klarheit? | Der US-Pharmariese verzeichnet eine minimale Kursstabilisierung nach dramatischen Verlusten, während UBS ihr Kursziel senkt und Analysten zunehmend skeptisch bleiben. Die Aktie des US-amerikanischen... ► Artikel lesen | |
| TEVA | 12,150 | +1,25 % | Teva Aktie: Gute Nachrichten, Tiefer Fall? | Während die amerikanische Gesundheitsbehörde das Biosimilar AVT06 als Eylea-Alternative untersucht, verzeichnet der Pharmawert erhebliche Kursverluste im Börsenhandel. Gute Nachrichten für Teva Pharmaceutical:... ► Artikel lesen | |
| ABBOTT LABORATORIES | 116,00 | +0,69 % | Abbott Laboratories-Aktie heute gut behauptet: Aktienwert steigt (116,7083 €) | Der Anteilsschein von Abbott Laboratories notiert am Donnerstag etwas fester. Der jüngste Kurs betrug 132,59 US-Dollar. Ein Kursgewinn in Höhe von 2,89 US-Dollar erfreut derzeit die Aktionäre von Abbott... ► Artikel lesen | |
| DERMAPHARM | 36,500 | -1,88 % | Dermapharm Aktie: Leichte Erholung vor Quartalszahlen | Trotz gemischter Signale bewerten Experten die Aktie positiv - Spannung vor den Q1-Zahlen im Mai. Die Dermapharm-Aktie zeigt sich zu Wochenbeginn stabil und legte gestern um 0,27% auf 36,65 Euro zu.... ► Artikel lesen | |
| DOCMORRIS | 21,780 | +2,01 % | BAADER BANK stuft DocMorris auf 'Reduce' | MÜNCHEN (dpa-AFX Analyser) - Die Baader Bank hat DocMorris von "Add" auf "Reduce" abgestuft und das Kursziel von 44 auf 19 Franken gesenkt. Analyst Volker Bosse begründete dies in einer am Mittwoch... ► Artikel lesen |
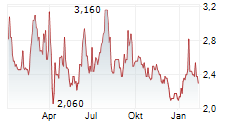
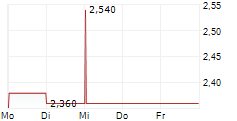
Das Unternehmen HUTCHMED CHINA LIMITED kann der Branche Pharma zugeordnet werden. Die relative Kursveränderung beträgt aktuell 0,00 % bei einem Aktienkurs von 2,360 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und .
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu HUTCHMED CHINA LIMITED finden Sie auf Wallstreet Online.