| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
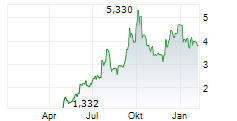
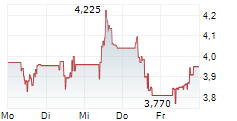
| 3,920 | 3,965 | 13:04 | |
| 3,925 | 3,960 | 06.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| IDORSIA Aktie jetzt für 0€ handeln | |||||
| Fr | FDA gibt grünes Licht für Idorsias Fabry-Mittel | 24 | cash | ||
| Fr | Idorsia Pharmaceuticals Ltd: Clear route to registration positions lucerastat as the potential first oral therapy for all patients with Fabry disease | 453 | GlobeNewswire (Europe) | Allschwil, Switzerland - February 06, 2026Idorsia Ltd (SIX: IDIA) announces the design of its FDA-agreed Phase 3 registration program for lucerastat in Fabry disease. Building on the robust biomarker... ► Artikel lesen | |
| 30.01. | Idorsia präsentiert Langzeitdaten zu Wirkstoff gegen Stoffwechselkrankheit | 28 | cash | ||
| 30.01. | Idorsia präsentiert Langzeitdaten zu Lucerastat bei Fabry-Krankheit auf Kongress | 20 | cash | ||
| 30.01. | Idorsia Pharmaceuticals Ltd: Idorsia to present long-term lucerastat data and kidney biopsy results at WORLDSymposium | 363 | GlobeNewswire (Europe) | Data from up to 42 months of treatment reinforce lucerastat's potential as a first-in-class oral substrate reduction therapy addressing key unmet needs across the Fabry populationThe company is finalizing... ► Artikel lesen | |
| 28.01. | Idorsia schliesst Lizenzvereinbarung für Quviviq in Lateinamerika ab | 32 | cash | ||
| 28.01. | Idorsia Pharmaceuticals Ltd: Global expansion of Idorsia's QUVIVIQ continues with EMS partnership for Latin America | 743 | GlobeNewswire (Europe) | Ad hoc announcement pursuant to Art. 53 LR
Regulatory dossier to obtain marketing authorization for QUVIVIQ has been submitted to ANVISA in Brazil
Allschwil, Switzerland - January 28, 2026Idorsia... ► Artikel lesen | |
| 12.01. | Idorsia Advances Fabry Disease Program With Encouraging Phase 3 Results | 20 | RTTNews | ||
| 12.01. | Idorsia kündigt neue Phase-3-Studie zu Lucerastat an | 29 | cash | ||
| 12.01. | Idorsia kündigt neue Phase-3-Studie zu Lucerastat bei Fabry-Krankheit an | 21 | cash | ||
| 12.01. | Idorsia Pharmaceuticals Ltd: Idorsia to present at J.P. Morgan 2026 Healthcare Conference | 459 | GlobeNewswire (Europe) | Allschwil, Switzerland - January 12, 2026Idorsia Ltd (SIX: IDIA) announces that Srishti Gupta, MD, Chief Executive Officer of Idorsia, will present at the 44th Annual J.P. Morgan Healthcare Conference... ► Artikel lesen | |
| 12.01. | Idorsia Pharmaceuticals Ltd: Nature Communications reports promising effect of Idorsia's lucerastat on kidney function in Fabry disease | 391 | GlobeNewswire (Europe) | In patients with impaired renal function or fast-deteriorating eGFR at baseline, lucerastat was associated with a marked attenuation of kidney function loss, suggesting a potential disease-modifying... ► Artikel lesen | |
| 06.01. | Idorsia Pharmaceuticals Ltd: Idorsia initiates a proof-of-concept trial with its oral first-in-class selective CCR6 antagonist | 522 | GlobeNewswire (Europe) | The trial aims to establish clinical proof-of-concept in psoriasis and proof-of-mechanism for other CCR6- and Th17-associated autoimmune indications
Allschwil, Switzerland - January 6, 2026Idorsia... ► Artikel lesen | |
| 05.01. | Idorsia Pharmaceuticals Ltd: Idorsia's daridorexant in women during menopausal transition age with insomnia | 2.786 | GlobeNewswire (Europe) | New analysis of a Phase 3 study of daridorexant shows that 50 mg improved both sleep and daytime functioning compared to placebo in women during menopausal transition ageBrigham and Women's Hospital... ► Artikel lesen | |
| 05.01. | Idorsia Pharmaceuticals Ltd: Idorsia's JERAYGO (aprocitentan) approved in Canada for the treatment of resistant hypertension | 619 | GlobeNewswire (Europe) | Idorsia receives approval from Health Canada for JERAYGO (aprocitentan) as the first and only endothelin receptor antagonist (ERA) for the treatment of resistant hypertension.JERAYGO is a new oral antihypertensive... ► Artikel lesen | |
| 17.12.25 | Idorsia: Struktureller Neustart: Für die heimische Biotech-Schmiede beginnt ein neues Kapitel. ... | 127 | payoff.ch | ||
| 10.12.25 | Idorsia Highlights New PRECISION Study Analysis Showing Strong Results For Aprocitentan | 24 | RTTNews | ||
| 10.12.25 | Idorsia Pharmaceuticals Ltd: New Hypertension publication underscores aprocitentan's potential in managing hypertension patients with CKD | 518 | GlobeNewswire (Europe) | New analysis from landmark Phase 3 PRECISION trial published in Hypertension highlights the renal-protective benefits of aprocitentan in addition to the marked reduction in blood pressure in a high-risk... ► Artikel lesen | |
| 09.12.25 | Idorsia Pharmaceuticals Ltd: Idorsia's treatment for insomnia disorder wins the inaugural Prix Galien Bridges Award in the 'Best Biotechnology & Pharmaceutical Product' category | 565 | GlobeNewswire (Europe) | Idorsia's dual orexin receptor antagonist, the first and only drug of its kind approved in Europe for the treatment of insomnia disorder, has been awarded the inaugural Prix Galien Bridges Award in... ► Artikel lesen | |
| 01.12.25 | Kepler Cheuvreux stuft Idorsia nach Umschuldung auf "Kaufen" hoch | 101 | Investing.com Deutsch |
1 2 3 4 Weiter >>
79 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 90,40 | +0,17 % | BioNTech- und Nvidia-Aktien verkauft, Medline und Alphabet rein: Erste 13F-Filings sind da! | Der britische Vermögensverwalter Baillie Gifford hat als einer der ersten sein 13F-Filing für das 4. Quartal abgegeben. Unter den großen Namen im Portfolio gibt es ein paar spannende Veränderungen.... ► Artikel lesen | |
| EVOTEC | 6,148 | +0,79 % | SDAX: Evotec-Aktie schießt zweistellig nach oben | Eine Kaufempfehlung von Berenberg entfacht neuen Optimismus bei Evotec und katapultiert die Biotech-Aktie an die Indexspitze. Die Aktie der Evotec hat am Dienstagmittag ein Kursfeuerwerk gezündet und... ► Artikel lesen | |
| BB BIOTECH | 50,60 | -0,78 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| MEDIGENE | 0,042 | -8,26 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 43,170 | -0,70 % | JEFFERIES stuft QIAGEN NV auf 'Buy' | NEW YORK (dpa-AFX Analyser) - Das Analysehaus Jefferies hat die Einstufung für Qiagen mit einem Kursziel von 57 US-Dollar auf "Buy" belassen. Umsatz und Ergebnis des vierten Quartals hätten die Erwartungen... ► Artikel lesen | |
| MODERNA | 34,650 | -0,07 % | Arbutus jumps on ruling in patent dispute with Moderna | ||
| PAION | 0,200 | +8,11 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WIRD GEPRUEFT | Fuer folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wird ein Mistrade-Antrag geprueft:ISIN Datum Zeit Volumen Preis Fair Value (AS)DE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 3,986 | +0,61 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| AMGEN | 325,15 | +0,06 % | Märkte am Morgen: "KI-Realitätscheck", Wolters Kluwer, AMD, Amgen, Walmart, Novo Nordisk | Die Stimmung an den Aktienmärkten ist derzeit sehr, sehr wechselhaft. Das war gestern deutlich zu spüren. Der DAX war bemerkenswert stark in den Handel gestartet, schloss allerdings leicht im Minus.... ► Artikel lesen | |
| EPIGENOMICS | 0,870 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 6,910 | -0,80 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| STRYKER | 302,10 | -0,33 % | Stryker Corporation: Stryker declares an $0.88 per share quarterly dividend | Portage, Michigan, Feb. 04, 2026 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) announced that its Board of Directors has declared a quarterly dividend of $0.88 per share payable April 30, 2026, to shareholders... ► Artikel lesen | |
| BIOGEN | 164,25 | +3,79 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,420 | -1,22 % | PTA-news: Biofrontera AG: Biofrontera berichtet über die Ergebnisse der ersten neun Monate des Jahres 2025 | DJ PTA-News: Biofrontera AG: BIOFRONTERA BERICHTET ÜBER DIE ERGEBNISSE DER ERSTEN NEUN MONATE DES JAHRES 2025
Unternehmensmitteilung für den Kapitalmarkt
Biofrontera AG: BIOFRONTERA... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,910 | 0,00 % | HEIDELBERG PHARMA AG zeigt klare Stärke im Trend |
Das Unternehmen IDORSIA AG kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell +0,13 % bei einem Aktienkurs von 3,950 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu IDORSIA AG finden Sie auf Wallstreet Online.