- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Di | INVENTIVA: Inventiva announces filing of its 2024 Universal Registration Document and 2024 Annual Report on Form 20-F | 566 | GlobeNewswire (Europe) | Daix (France), New York City (New York, United States), April 15, 2025 - Inventiva (Euronext Paris and Nasdaq: IVA) ("Inventiva" or the "Company"), a clinical-stage biopharmaceutical company focused... ► Artikel lesen | |
| Di | Inventiva S.A. - 20-F, Annual and transition report of foreign private issuers | 2 | SEC Filings | ||
| 11.04. | Inventiva S.A. - 6-K, Report of foreign issuer | 3 | SEC Filings | ||
| 04.04. | Guggenheim cuts Inventiva stock target to $9, maintains Buy rating | 1 | Investing.com | ||
| 02.04. | Inventiva stock rises following NATiV3 trial enrollment completion | 2 | Investing.com | ||
| INVENTIVA SA ADR Aktie jetzt für 0€ handeln | |||||
| 02.04. | Inventiva-Aktie steigt nach Abschluss der NATiV3-Studienrekrutierung | 1 | Investing.com Deutsch | ||
| 02.04. | Stifel maintains Buy on Inventiva shares with $17 target | 3 | Investing.com | ||
| 01.04. | INVENTIVA: Inventiva announces completion of enrollment in the Phase 3 NATiV3 clinical trial of lanifibranor in patients with MASH and advanced fibrosis | 368 | GlobeNewswire (Europe) | Target enrollment exceeded with 1009 patients randomized in the main cohort and 410 patients in the exploratory cohort Topline results from NATiV3 projected in the second half of 2026 and, if positive... ► Artikel lesen | |
| 26.03. | Inventiva S.A GAAP EPS of -€3.08 misses by €1.27, revenue of €9.19M beats by €1.88M | 1 | Seeking Alpha | ||
| 26.03. | Inventiva S.A. - 6-K, Report of foreign issuer | 1 | SEC Filings | ||
| 26.03. | INVENTIVA: Inventiva reports its 2024 full year results and provides a business update | 397 | GlobeNewswire (Europe) | Revenues of €9.2 million for the full year of 2024Cash and cash equivalents at €96.6 million as of December 31, 2024 First tranche of up to €348 million Structured Financing closed with aggregate gross... ► Artikel lesen | |
| 19.03. | INVENTIVA: Inventiva announces the schedule of publication and presentation of its 2024 Full-Year Financial Results | 858 | GlobeNewswire (Europe) | Daix (France), New York City (New York, United States), March 19, 2025 - Inventiva (Euronext Paris and NASDAQ: IVA) ("Inventiva" or the "Company"), a clinical-stage biopharmaceutical company focused... ► Artikel lesen | |
| 02.03. | Inventiva Aktie: Prognosen unter nüchterner Betrachtung | 562 | Börse Global | Die französische Biotechnologiefirma Inventiva verzeichnete am 28. Februar 2025 einen Kursrückgang von 3,80% auf 2,91 EUR. Trotz dieser kurzfristigen Schwäche konnte das Unternehmen im vergangenen Monat... ► Artikel lesen | |
| 26.02. | INVENTIVA: Inventiva announces the publication in Biomedicine & Pharmacotherapy of the results from a preclinical study showing improvement of portal hypertension with lanifibranor treatment | 343 | GlobeNewswire (Europe) | The study demonstrated that lanifibranor improved Portal Hypertension (PH) in mouse models of fibrotic PH and prehepatic non-fibrotic PHLanifibranor was observed to decrease portal pressure by improving... ► Artikel lesen | |
| 20.02. | INVENTIVA: Inventiva and Hepalys Pharma, Inc. announce the initiation of the clinical development program of lanifibranor in Japan with the dosing of the first participant in Phase 1 trial | 352 | GlobeNewswire (Europe) | Initiation of the clinical development program of lanifibranor in Japan with the dosing of the first participant in Phase 1 studyPositive results could support the initiation of a pivotal Phase 3 trial... ► Artikel lesen | |
| 20.02. | Inventiva S.A. - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 11.02. | Inventiva lays off 50% of staff to go all-in on MASH drug | 3 | FierceBiotech | ||
| 10.02. | INVENTIVA: Inventiva reports preliminary 2024 fiscal year financial results and provides a business update | 344 | GlobeNewswire (Europe) | Revenues of €9.2 million for the full year of 2024Cash and cash equivalents at €96.6 million as of December 31, 2024 First tranche of up to €348 million structured financing closed with aggregate gross... ► Artikel lesen | |
| 10.02. | Inventiva S.A. - 6-K, Report of foreign issuer | - | SEC Filings | ||
| 29.01. | INVENTIVA: Inventiva announces the publication of the results from the investigator-initiated proof-of-concept clinical trial evaluating lanifibranor in patients with T2D and MASLD in the Journal of Hepatology | 288 | GlobeNewswire (Europe) | As previously reported1, the study met the primary efficacy endpoint for the treatment with lanifibranor 800mg demonstrating a 44% reduction of hepatic fat measured by proton magnetic resonance spectroscopy... ► Artikel lesen |
Seite:
1 2 3 Weiter >>
53 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| AURORA CANNABIS | 3,860 | -1,28 % | Aurora Cannabis Inc (3): Aurora Cannabis offers inhalers to U.K. patients | ||
| CANOPY GROWTH | 1,052 | +0,77 % | Canopy Growth-Aktie: Gleich zwei neue Gefahren | Die Kursentwicklung der Canopy Growth-Aktie ist seit langer Zeit ein einziges Trauerspiel. In den letzten zwölf Monaten lösten sich 90% des Unternehmenswertes in Luft auf und seit einer Woche notiert... ► Artikel lesen | |
| TILRAY BRANDS | 0,400 | +0,78 % | Tilray Brands, Inc.: Tilray Brands Announces Proposed Reverse Stock Split and Corresponding Special Meeting of Stockholders | NEW YORK and LEAMINGTON, Ontario, April 17, 2025 (GLOBE NEWSWIRE) -- Tilray Brands, Inc. ("Tilray" or "Company") (Nasdaq: TLRY; TSX: TLRY), a global lifestyle and consumer packaged goods company at... ► Artikel lesen | |
| TEVA | 12,150 | +1,25 % | Teva Aktie: Gute Nachrichten, Tiefer Fall? | Während die amerikanische Gesundheitsbehörde das Biosimilar AVT06 als Eylea-Alternative untersucht, verzeichnet der Pharmawert erhebliche Kursverluste im Börsenhandel. Gute Nachrichten für Teva Pharmaceutical:... ► Artikel lesen | |
| VERTEX PHARMACEUTICALS | 432,00 | +0,48 % | Vertex Gains European Approval For Expanded Use Of KAFTRIO In Treating Cystic Fibrosis | CAMBRIDGE (MASSACHUSETTS) (dpa-AFX) - Vertex Pharmaceuticals (VRTX) announced that the European Commission has approved a label expansion for KAFTRIO (ivacaftor/tezacaftor/elexacaftor) in combination... ► Artikel lesen | |
| ARZNEIWERK AG VIDA | 0,545 | +3,81 % | PTA-Adhoc: Arzneiwerk AG VIDA: Keine Verpflichtung für die Erstellung eines Konzernabschlusses | DJ PTA-Adhoc: Arzneiwerk AG VIDA: Keine Verpflichtung für die Erstellung eines Konzernabschlusses
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Arzneiwerk AG VIDA: Keine... ► Artikel lesen | |
| CRONOS GROUP | 1,523 | -1,81 % | Cronos Group Inc.: Cronos Appoints Anna Shlimak as Chief Financial Officer | TORONTO, March 19, 2025 (GLOBE NEWSWIRE) -- Cronos Group Inc. (NASDAQ: CRON) (TSX: CRON) ("Cronos" or the "Company"), an innovative global cannabinoid company, today announced the appointment of Anna... ► Artikel lesen | |
| VIATRIS | 6,668 | +0,36 % | Aktie von Viatris heute am Aktienmarkt kaum gefragt: Kurs fällt (6,5842 €) | Im US-amerikanischen Wertpapierhandel liegt der Anteilsschein von Viatris aktuell im Minus. Der jüngste Kurs betrug 7,42 US-Dollar. Am US-amerikanischen Aktienmarkt hat sich heute die Viatris-Aktie... ► Artikel lesen | |
| EYEPOINT PHARMACEUTICALS | 4,778 | -4,25 % | EyePoint Pharmaceuticals, Inc.: EyePoint Pharmaceuticals Reports Inducement Grants Under NASDAQ Listing Rule 5635(c)(4) | WATERTOWN, Mass., April 16, 2025 (GLOBE NEWSWIRE) -- EyePoint Pharmaceuticals, Inc. (Nasdaq: EYPT), a company committed to developing and commercializing therapeutics to help improve the lives of... ► Artikel lesen | |
| INCYTE | 51,16 | -0,04 % | Incyte Earnings Preview: What to Expect | ||
| BAUSCH HEALTH | 4,555 | +1,47 % | Bausch Health Companies Inc.: U.S. District Court Grants Summary Judgment in Favor of FDA, Salix, and Teva, and Against Norwich | LAVAL, QC / ACCESS Newswire / April 17, 2025 / Bausch Health Companies Inc. (NYSE:BHC)(TSX:BHC) (the "Company" or "Bausch Health"), and its gastroenterology business Salix Pharmaceuticals, Inc., today... ► Artikel lesen | |
| EDWARDS LIFESCIENCES | 63,27 | +0,56 % | Edwards Lifesciences Corporation: Edwards SAPIEN M3 Receives CE Mark, Becoming World's First Transfemoral Transcatheter Mitral Valve Replacement System | Edwards Lifesciences Corporation (NYSE: EW) today announced the company's SAPIEN M3 mitral valve replacement system received CE Mark for the transcatheter treatment of patients with symptomatic (moderate-to-severe... ► Artikel lesen | |
| CRESCO LABS | 0,570 | -1,56 % | Cresco Labs Aktie: Komplexe Lage droht? | Cresco Labs verzeichnete im Geschäftsjahr 2024 eine beeindruckende finanzielle Entwicklung mit einem Jahresumsatz von 724 Millionen Euro. Trotz dieser positiven Kennzahlen schloss das Unternehmen mit... ► Artikel lesen | |
| IONIS PHARMACEUTICALS | 24,840 | -0,52 % | Ionis Aktie: Alles auf den Punkt gebracht | Ionis Pharmaceuticals verstärkt Zusammenarbeit mit Sobi und fördert weltweite Olezarsen-Vermarktung außerhalb der USA. Ionis Pharmaceuticals stärkt seine Position im Biotechnologiesektor durch strategische... ► Artikel lesen |
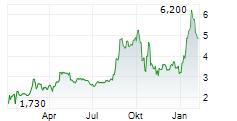
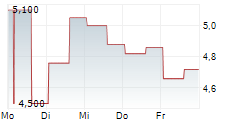
INVENTIVA SA ADR gehört der Branche Pharma an. Die relative Kursveränderung beträgt aktuell +0,71 % bei einem Aktienkurs von 2,820 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und .
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu INVENTIVA SA ADR finden Sie auf Wallstreet Online.