- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| INVIVYD Aktie jetzt für 0€ handeln | |||||
| Mi | H.C. Wainwright reiterates Buy rating on Invivyd stock amid FDA feedback | 3 | Investing.com | ||
| Di | Invivyd rises as FDA agrees with late-stage trial design for COVID-19 therapy | 3 | Seeking Alpha | ||
| Di | Invivyd: FDA gibt Vorgaben für Vergleichsstudie von COVID-Antikörper und mRNA-Impfstoff | 8 | Investing.com Deutsch | ||
| Di | Invivyd Aligns with the U.S. FDA on LIBERTY, a Phase 3 Trial to Evaluate the Safety of VYD2311 Antibody Versus mRNA COVID Vaccine, and to Characterize the Safety and Immunology of Antibody and Vaccine Co-Administration | 93 | GlobeNewswire (Europe) | LIBERTY is part of the Company's broader REVOLUTION clinical program designed to elaborate the profile of monoclonal antibody-mediated prophylaxis from COVID-19 and the potential medical benefits... ► Artikel lesen | |
| 23.01. | H.C. Wainwright bekräftigt Kaufempfehlung für Invivyd vor wichtigen Studiendaten | 7 | Investing.com Deutsch | ||
| 23.01. | H.C. Wainwright reaffirms Buy rating on Invivyd stock ahead of key trial data | 1 | Investing.com | ||
| 22.01. | Invivyd teams up with Olympic skier Lindsey Vonn for antibody education | 1 | FiercePharma | ||
| 22.01. | Invivyd: Partnerschaft mit Ski-Olympiasiegerin Lindsey Vonn treibt Aktie an | 1 | Investing.com Deutsch | ||
| 22.01. | Invivyd partners with ski champion Lindsey Vonn on antibody education | 1 | Investing.com | ||
| 22.01. | Invivyd Announces Partnership with World Champion Skier Lindsey Vonn to Educate Americans on Antibodies and Disease Prevention | 1 | GlobeNewswire (USA) | ||
| 21.01. | Invivyd: BTIG bestätigt Kaufempfehlung wegen Potenzial bei Long-COVID-Behandlung | 4 | Investing.com Deutsch | ||
| 21.01. | Invivyd stock maintains Buy rating at BTIG on Long COVID treatment potential | 2 | Investing.com | ||
| 20.01. | Invivyd and SPEAR Study Group Announce Plan for Phase 2 Study of VYD2311 for Treatment of Long COVID and COVID Vaccine-Injured Individuals to Commence Mid-2026 | 172 | GlobeNewswire (Europe) | Phase 2 clinical trial will evaluate the safety, translational biology, and exploratory clinical efficacy of VYD2311 in people with Long COVID or COVID vaccine injuryParticipants to include people... ► Artikel lesen | |
| 08.01. | Invivyd: PEMGARDA-Umsatz steigt im vierten Quartal um 25 % auf 17,2 Mio. US-Dollar | 2 | Investing.com Deutsch | ||
| 08.01. | Invivyd reports 25% growth in Q4 PEMGARDA revenue to $17.2 million | 1 | Investing.com | ||
| 08.01. | Invivyd Reports Preliminary Fourth Quarter 2025 Revenue and Recent Business Highlights | 136 | GlobeNewswire (Europe) | Preliminary Q4 2025 PEMGARDA® (pemivibart) net product revenue of $17.2 million, representing 25% growth year-over-year and 31% growth quarter-over-quarterPreliminary ending 2025 cash and cash equivalents... ► Artikel lesen | |
| 08.01. | Invivyd, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 23.12.25 | Invivyd granted FDA fast track status for COVID therapy | 1 | Seeking Alpha | ||
| 23.12.25 | FDA grants fast track designation for Invivyd's COVID antibody | 1 | Investing.com | ||
| 23.12.25 | Invivyd-Aktie legt zu: FDA beschleunigt Zulassungsverfahren für Covid-Antikörper | 7 | Investing.com Deutsch |
1 2 3 4 5 Weiter >>
90 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 90,40 | +0,17 % | Startschuss! BioTech-Sektor profitiert von Rotation! Augen auf bei Evotec, Bayer, Vidac Pharma and BioNTech | Mit positiver Grundstimmung hat die Börse das Jahr 2026 eingeläutet. Dass Renditechancen nicht allein im Technologiesektor liegen, bewies zuletzt der Minen- und Rohstoffbereich, wo etliche Titel Kursverdopplungen... ► Artikel lesen | |
| EVOTEC | 6,148 | +0,79 % | SDAX: Evotec-Aktie schießt zweistellig nach oben | Eine Kaufempfehlung von Berenberg entfacht neuen Optimismus bei Evotec und katapultiert die Biotech-Aktie an die Indexspitze. Die Aktie der Evotec hat am Dienstagmittag ein Kursfeuerwerk gezündet und... ► Artikel lesen | |
| BB BIOTECH | 50,60 | -0,78 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| MEDIGENE | 0,042 | -8,26 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 43,170 | -0,70 % | Ionos, Kontron, Lanxess, Qiagen, Redcare Pharmacy, Siltronic, TeamViewer: Neue Aktien-Positionen der Short-Seller | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| MODERNA | 34,650 | -0,07 % | Arbutus jumps on ruling in patent dispute with Moderna | ||
| PAION | 0,200 | +8,11 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WIRD GEPRUEFT | Fuer folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wird ein Mistrade-Antrag geprueft:ISIN Datum Zeit Volumen Preis Fair Value (AS)DE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 3,986 | +0,61 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| AMGEN | 325,15 | +0,06 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| EPIGENOMICS | 0,870 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 6,910 | -0,80 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| BIOGEN | 164,25 | +3,79 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,420 | -1,22 % | PTA-news: Biofrontera AG: Biofrontera berichtet über die Ergebnisse der ersten neun Monate des Jahres 2025 | DJ PTA-News: Biofrontera AG: BIOFRONTERA BERICHTET ÜBER DIE ERGEBNISSE DER ERSTEN NEUN MONATE DES JAHRES 2025
Unternehmensmitteilung für den Kapitalmarkt
Biofrontera AG: BIOFRONTERA... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,910 | 0,00 % | HEIDELBERG PHARMA AG zeigt klare Stärke im Trend | ||
| ILLUMINA | 101,04 | -0,18 % | Illumina-Aktie -10%: Kann man hier günstig einsteigen? | Mit einem Kursverlust von -10% war die Illumina-Aktie am Freitag der große Verlierer im S&P 500. Der Hersteller von Gensequenzierungsmaschinen setzte damit seinen Abwärtstrend der letzten Tage fort.... ► Artikel lesen |
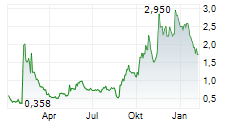
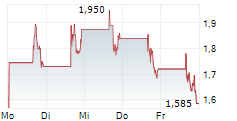
INVIVYD INC gehört der Branche Biotechnologie an. Der aktuelle Kurs liegt bei 1,585 Euro und damit -7,85 % im Minus.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu INVIVYD INC finden Sie auf Wallstreet Online.