| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 5,400 | 5,750 | 16:23 | |
| 5,400 | 5,600 | 16:21 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mi | HSI Closes at 23,202, Down 4 pts; HSTI Closes at 5,426, Up 19 pts; CHOW TAI FOOK Up over 10%; WH GROUP, LEAPMOTOR, KEYMED BIO-B, CZBANK, CQRC BANK Hit New Highs; Market Turnover Rises | 7 | AASTOCKS | ||
| Mi | HSI Closes Midday at 23,221, Up 14 pts; HSTI Closes Midday at 5,438, Up 31 pts; CHOW TAI FOOK Up over 5%; LEAPMOTOR, CHINA EAST EDU, CQRC BANK, CZBANK, KEYMED BIO-B Hit New Highs | 3 | AASTOCKS | ||
| 26.03. | Keymed Biosciences Announces Annual Results of 2024 | 85 | PR Newswire | CHENGDU, China, March 26, 2025 /PRNewswire/ -- March. 25, 2025, Keymed Biosciences Inc. (HKEX: 02162) announced its annual results of 2024, along with a corporate... ► Artikel lesen | |
| 24.03. | KEYMED BIO-B (02162): TERMS OF REFERENCE OF THE NOMINATION COMMITTEE | 1 | HKEx | ||
| KEYMED BIOSCIENCES Aktie jetzt für 0€ handeln | |||||
| 24.03. | KEYMED BIO-B (02162): LIST OF DIRECTORS AND THEIR ROLES AND FUNCTIONS | - | HKEx | ||
| 24.03. | KEYMED BIO-B (02162): ANNUAL RESULTS ANNOUNCEMENT FOR THE YEAR ENDED DECEMBER 31, 2024; AND CHANGE OF COMPOSITION OF THE NOMINATION COMMITTEE | 1 | HKEx | ||
| 12.03. | KEYMED BIO-B (02162): DATE OF BOARD MEETING | 2 | HKEx | ||
| 10.02. | Keymed's Stapokibart approved by NMPA for seasonal allergic rhinitis | 1 | Pharmaceutical Technology | ||
| 08.02. | KeyMed Biosciences' Stapokibart Approved In China For Seasonal Allergic Rhinitis Treatment | 2 | RTTNews | ||
| 08.02. | Keymed Biosciences Inc.: Keymed Biosciences Announces Approval of Stapokibart For the Treatment of Seasonal Allergic Rhinitis | 625 | PR Newswire | CHENGDU, China, Feb. 8, 2025 /PRNewswire/ -- Keymed Biosciences (HKEX: 02162) today announced the National Medical Products Administration (the "NMPA") of China has recently approved the... ► Artikel lesen | |
| 07.02. | KEYMED BIO-B (02162): VOLUNTARY ANNOUNCEMENT NMPA APPROVED THE SNDA OF STAPOKIBART FOR THE TREATMENT OF SEASONAL ALLERGIC RHINITIS | 2 | HKEx | ||
| 21.01. | InnoCare, Keymed sell ex-China rights for bispecific to RTW-built biotech in $520M deal | 1 | FierceBiotech | ||
| 21.01. | China's KeyMed, InnoCare Grant Prolium Bio Global Rights to Cancer Drug for USD520 Million | 1 | Yicai Global | ||
| 21.01. | BRIEF: InnoCare, KeyMed in antibody licensing agree with Prolium | 1 | Bamboo Works | ||
| 20.01. | KeyMed partners with US-based biotech Prolium in bispecific antibody deal | 1 | Pharmaceutical Technology | ||
| 20.01. | KEYMED BIO-B (02162): VOLUNTARY ANNOUNCEMENT LICENSE AGREEMENT WITH PROLIUM FOR THE DEVELOPMENT AND COMMERCIALIZATION OF CM355 (ICP-B02) | 1 | HKEx | ||
| 10.01. | Biotech creator and China's Keymed launch antibody company with $180M series A | 1 | FierceBiotech | ||
| 10.01. | KEYMED BIO-B (02162): VOLUNTARY ANNOUNCEMENT IN RELATION TO THE LICENSE AGREEMENT FOR CM313 | 1 | HKEx | ||
| 23.12.24 | Keymed Biosciences Announces Approval Of Stapokibart For The Treatment Of Chronic Rhinosinusitis With Nasal Polyposis | 266 | PR Newswire | CHENGDU, China, Dec. 23, 2024 /PRNewswire/ -- Keymed Biosciences Inc. (HKEX: 02162) today announced the National Medical Products Administration (the "NMPA") of China has recently approved... ► Artikel lesen | |
| 23.12.24 | KEYMED BIO-B (02162): VOLUNTARY ANNOUNCEMENT NMPA APPROVED THE SNDA OF STAPOKIBART FOR THE TREATMENT OF CHRONIC RHINOSINUSITIS WITH NASAL POLYPOSIS | 2 | HKEx |
Seite:
1 2 Weiter >>
28 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| QIAGEN | 35,530 | -1,85 % | Biotech Report: Qiagen behauptet, Evotec und Biontech rutschen ab | (shareribs.com) Frankfurt / New York 28.03.2025 - Die Biotechnologiewerte entwickeln sich im deutschen Handel uneinheitlich. Für Biontech geht es deutlich abwärts. Auch in den USA zeigt sich der Handel... ► Artikel lesen | |
| BIONTECH | 80,05 | -4,30 % | BioNTech-Aktie: Das tragische Ende! | News von Trading-Treff.de Für die BioNTech sieht es nach einem tragischen Ende des Aufwärtsmarsches aus. Die Mainzer haben auch am Donnerstag einen Abschlag in Höhe von mehr als -1,9 % hinnehmen müssen.... ► Artikel lesen | |
| EVOTEC | 5,545 | -6,02 % | Aktie vor 100 %-Rally! Evotec, thyssenkrupp, Vidac Pharma Aktie vor goldenem April? | Können sich Aktionäre bald über 100 % Kursgewinn freuen? Geht es nach Analysten, könnte dies bei Evotec bevorstehen. Allerdings müssen Aktionäre zuvor einen wichtigen Termin im April markieren. Dort... ► Artikel lesen | |
| TEMPUS AI | 43,210 | -6,51 % | Tempus AI Surges 43% YTD: Is it the Right Time to Invest in the Stock? | ||
| BB BIOTECH | 29,550 | -2,96 % | BB Biotech Aktie: Gibt es jetzt Probleme? | Die BB Biotech AG kämpft aktuell mit einem erheblichen Wertverlust. Die Aktie notiert bei 31,85 CHF und hat innerhalb der letzten Woche einen Rückgang von 8,08% verzeichnet. Im Monatsvergleich fällt... ► Artikel lesen | |
| STRYKER | 323,20 | -3,23 % | Dividendenbekanntmachungen (31.03.2025) | Unternehmen ISIN-Code Dividende (Währung) Dividende (EUR) ACADIA REALTY TRUST US0042391096 0,2 USD 0,1847 EUR ACADIAN TIMBER CORP CA0042721005 0,29 CAD 0,187 EUR ADVANCED FLOWER CAPITAL... ► Artikel lesen | |
| VALNEVA | 2,640 | -16,61 % | Commerzbank, Hensoldt, Rheinmetall, Steyr Motors, ThyssenKrupp, Valneva - 4investors Aktien Top-News | Wo ist am Aktienmarkt etwas los, welche Themen interessieren Anleger derzeit besonders? Vor allem für Trader ist es wichtig zu wissen, wo "die Musik spielt" und welche Themen an der Börse aktuell besonders... ► Artikel lesen | |
| AMGEN | 278,35 | -0,98 % | Amgen: Uplizna (inebilizumab-cdon) Is Now The First And Only FDA-approved Treatment For Igg4-related Disease | Breakthrough CD19+ B-Cell Targeted Therapy Delivered an 87% Reduction in the Risk of Flares Versus Placebo
UPLIZNA Shown to Deliver Corticosteroid-Free, Flare-Free... ► Artikel lesen | |
| MODERNA | 22,550 | -3,30 % | Impfstoff-Aktien Moderna, BioNTech und Co massiv unter Druck - Gegenwind in den USA | Auch zum Start in die neue Handelswoche bahnen sich erneut Kursverluste bei einigen US-gelisteten Aktien aus dem Pharma- und Biotech-Sektor an. Vor allem bei den Impfstoff-Titeln geht es teilweise kräftig... ► Artikel lesen | |
| CUREVAC | 2,402 | -6,90 % | CureVac-Aktie: Donnerschlag! | News von Trading-Treff.de Die CureVac-Aktie ist am Donnerstag um rund 1,2 % gestiegen. Am Vorabend war es in den USA an der Nasdaq bereits um mehr als 5 % aufwärts gegangen. Dabei hat die Aktie aus... ► Artikel lesen | |
| HARMONY BIOSCIENCES | 30,960 | 0,00 % | Pre-market Movers: ALTI, CXAI, PAMT, HRMY, ICCT. | ROME (dpa-AFX) - The following are some of the stocks making big moves in Friday's pre-market trading (as of 09.15 A.M. ET).In the Green CXApp Inc (CXAI) is up over 37% at $1.11.
PAM Transportation... ► Artikel lesen | |
| JANUX THERAPEUTICS | 27,010 | 0,00 % | Pre-market Movers: Cheetah Net Supply Chain Service, NLS Pharmaceutics, PSQ Holdings, Janux Therapeutics, Coherus BioSciences | CANBERA (dpa-AFX) - The following are some of the stocks making big moves in Tuesday's pre-market trading (as of 08.45 A.M. ET).In the Green Cheetah Net Supply Chain Service Inc. (CTNT) is up... ► Artikel lesen | |
| GUBRA | 51,20 | -8,24 % | AKTIONÄR-Depotwert Gubra fällt und fällt: Kaufempfehlung verpufft | Der schwache Gesamtmarkt und die mehrmonatige Schwäche des großen Wettbewerbers Novo Nordisk setzen die Aktie des dänischen Wirkstoffforschers Gubra weiter unter Druck. Gute Neuigkeiten von Unternehmensseite... ► Artikel lesen | |
| BEAM THERAPEUTICS | 15,490 | -5,69 % | Biotech stocks plunge as FDA's Peter Marks resigns over RFK Jr. dispute, Moderna and Beam Therapeutics hit hard | ||
| TELIX PHARMACEUTICALS | 12,305 | -14,01 % | Telix Pharmaceuticals Limited: Telix Announces First Patient Dosed in First-in-Human ZOLAR Trial of TLX300-CDx in Advanced Soft Tissue Sarcoma | MELBOURNE, Australia, April 02, 2025 (GLOBE NEWSWIRE) -- Telix Pharmaceuticals Limited (ASX: TLX; NASDAQ: TLX, Telix, the Company) today announces that a first patient has been dosed in the Phase... ► Artikel lesen |
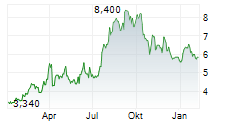
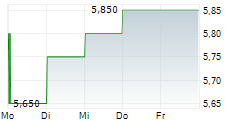
Das Unternehmen KEYMED BIOSCIENCES INC kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell +2,83 % bei einem Aktienkurs von 5,450 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und .
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu KEYMED BIOSCIENCES INC finden Sie auf Wallstreet Online.