- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 27.01. | LUYE PHARMA's Nanjing Luye Receives RMB285M Capital Injection | 2 | AASTOCKS | ||
| 27.01. | LUYE PHARMA (02186): DISCLOSEABLE AND CONNECTED TRANSACTION CAPITAL INJECTION IN NANJING LUYE | 1 | HKEx | ||
| 27.01. | LUYE PHARMA (02186): DISCLOSEABLE TRANSACTIONS AND CONNECTED TRANSACTION FURTHER ANNOUNCEMENT ON THE TRANSFER OF 25% INTEREST IN NANJING LUYE AND RELATED ... | 1 | HKEx | ||
| 08.01. | LUYE PHARMA (02186): VOLUNTARY ANNOUNCEMENT NEW DRUG APPLICATION FOR CLASS 1 INNOVATIVE DRUG RUOXINLIN FOR THE NEW INDICATION OF GENERALIZED ANXIETY DISORDER ... | 2 | HKEx | ||
| 31.12.25 | LUYE PHARMA (02186): TERMS OF REFERENCE FOR NOMINATION COMMITTEE OF THE BOARD OF DIRECTORS OF THE COMPANY | 1 | HKEx | ||
| 31.12.25 | LUYE PHARMA (02186): LIST OF DIRECTORS AND THEIR ROLE AND FUNCTION | 1 | HKEx | ||
| LUYE PHARMA Aktie jetzt für 0€ handeln | |||||
| 31.12.25 | LUYE PHARMA (02186): APPOINTMENT OF NOMINATION COMMITTEE MEMBER | 1 | HKEx | ||
| 24.12.25 | Luye Pharma Grants Nhwa Rights To Commercialize Three LAI Antipsychotics In Chinese Mainland | 2 | RTTNews | ||
| 24.12.25 | LUYE PHARMA (02186): VOLUNTARY ANNOUNCEMENT THE GRANT OF EXCLUSIVE COMMERCIALIZATION RIGHTS TO NHWA FOR THREE LONG-ACTING INJECTABLE ANTIPSYCHOTICS IN ... | 4 | HKEx | ||
| 12.12.25 | LUYE PHARMA (02186): DISCLOSEABLE TRANSACTION COMPLETION OF THE ISSUE OF EXCHANGEABLE PREFERENCE SHARES BY A WHOLLY-OWNED SUBSIDIARY OF THE COMPANY | 2 | HKEx | ||
| 24.11.25 | LUYE PHARMA (02186): VOLUNTARY ANNOUNCEMENT APPROVAL OBTAINED FOR THE GROUP'S NEW DRUG LY03017 FOR CLINICAL TRIALS IN THE U.S. | 2 | HKEx | ||
| 21.11.25 | LUYE PHARMA (02186): DISCLOSEABLE TRANSACTION ISSUE OF EXCHANGEABLE PREFERENCE SHARES BY A WHOLLY-OWNED SUBSIDIARY OF THE COMPANY | 1 | HKEx | ||
| 29.09.25 | LUYE PHARMA (02186): INTERIM REPORT 2025 | 5 | HKEx | ||
| 09.09.25 | LUYE PHARMA (02186): NEXT DAY DISCLOSURE RETURN | 2 | HKEx | ||
| 09.09.25 | LUYE PHARMA (02186): OVERSEAS REGULATORY ANNOUNCEMENT | 2 | HKEx | ||
| 28.08.25 | LUYE PHARMA (02186): ANNOUNCEMENT OF INTERIM RESULTS FOR THE SIX MONTHS ENDED 30 JUNE 2025 | 5 | HKEx | ||
| 18.08.25 | LUYE PHARMA (02186): NOTICE OF BOARD MEETING | 4 | HKEx | ||
| 18.08.25 | LUYE PHARMA (02186): VOLUNTARY ANNOUNCEMENT PATIENT ENROLLMENT COMPLETED FOR THE PHASE III CLINICAL STUDY OF RUOXINLIN IN CHINA FOR TREATING GENERALIZED ... | 3 | HKEx | ||
| 14.08.25 | LUYE PHARMA (02186): OVERSEAS REGULATORY ANNOUNCEMENT | 1 | HKEx | ||
| 14.08.25 | LUYE PHARMA (02186): NEXT DAY DISCLOSURE RETURN | - | HKEx |
20 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 45,800 | +2,20 % | Dax - Ausblick auf die neue Handelswoche: Bayer - Wann fällt die Marke von 50 Euro? | Nach einer überaus turbulenten Handelswoche richten sich die Blicke bereits auf die neue Handelswoche und damit auf den neuen Monat. Der Januar gestaltete sich für den Dax durchaus ambivalent. Dem Index... ► Artikel lesen | |
| NOVO NORDISK | 40,310 | +0,01 % | Abnehmpille schlägt ein: Starker Start ins neue Jahr - Novo Nordisk zeigt Eli Lilly die Rücklichter! | © Foto: Kristian Tuxen Ladegaard Berg - NurPhotoNach einem schwachen Vorjahr meldet sich Novo Nordisk 2026 eindrucksvoll zurück. Die neue Wegovy-Tablette sorgt in den USA für einen starken Marktstart.... ► Artikel lesen | |
| PFIZER | 23,015 | -0,07 % | Opening Bell: Gold/Silber, PepsiCo, Merck & Co, Pfizer, PayPal, Teradyne, Uber/Snap/Alphabet, Nvidia | Die US-Börsen sind zum Wochenstart fester aus dem Handel gegangen. Der Dow Jones gewann 1,05 Prozent, der Nasdaq Composite legte 0,56 Prozent zu. Am Freitag hatte die mögliche Nominierung von Kevin... ► Artikel lesen | |
| NOVARTIS | 132,06 | +0,03 % | Novartis macht über 17 Milliarden Gewinn | Basel - Novartis hat im Geschäftsjahr 2025 einen Gewinn von 17,4 Milliarden US-Dollar verbucht, wie der Pharmakonzern am Mittwoch mitteilte. Das entspricht einer Steigerung von 11 Prozent zum Vorjahr.... ► Artikel lesen | |
| MERCK KGAA | 121,45 | -0,49 % | BARCLAYS stuft MERCK KGAA auf 'Equal Weight' | LONDON (dpa-AFX Analyser) - Die britische Investmentbank Barclays hat die Einstufung für Merck KGaA mit einem Kursziel von 130 Euro auf "Equal Weight" belassen. Der Fokus liege auf möglichen Risiken... ► Artikel lesen | |
| GILEAD SCIENCES | 128,98 | -0,05 % | Gilead wins favorable U.S. label update for Yescarta | ||
| AURORA CANNABIS | 2,925 | -0,85 % | Aurora Cannabis Inc.: Aurora Cannabis Announces Fiscal 2026 Third Quarter Results | NASDAQ | TSX: ACB
Expands YoY Total Net Revenue1 by 7% to $94.2 million, with Global Medical Cannabis Net Revenue1 Increasing by 12% to a Record $76.2 million
... ► Artikel lesen | |
| SANOFI | 80,49 | +0,05 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| GSK | 25,300 | +1,12 % | GSK: Besser als erwartet | Der Pharmakonzern konnte ein unerwartet starkes Schlussquartal vorweisen und damit besser abschneiden als gedacht. Der Umsatz von GSK klettert im vergangenen Jahr um 4 % auf knapp 32,7 Mrd. Pfund, währungsbereinigt... ► Artikel lesen | |
| ABBVIE | 189,20 | +0,11 % | Aktien New York Ausblick: Keine Tech-Erholung - Eli Lilly steigen kräftig | NEW YORK (dpa-AFX) - Mit Blick auf den Technologiesektor dürften sich die Investoren an den US-Börsen am Mittwoch zurückhalten. Der Nasdaq 100 Index, der am Vortag deutlich gefallen war, dürfte sich... ► Artikel lesen | |
| CANOPY GROWTH | 0,935 | -0,95 % | Canopy Growth: Treffer ohne Gewinn - Trading-Tipp wird zurückgezogen | ||
| ROCHE | 391,10 | -0,29 % | DZ BANK stuft ROCHE HOLDINGS AG auf 'Kaufen' | FRANKFURT (dpa-AFX Analyser) - Die DZ Bank hat den fairen Aktienwert für Roche von 324 auf 395 Franken angehoben und die Einstufung auf "Kaufen" belassen. "Die Pharma-Sparte hat den großen, nahezu parallelen... ► Artikel lesen | |
| STADA ARZNEIMITTEL | - | - | Pharmakonzern Stada: Nachträglich mehr Geld für Aktionäre | Hartnäckigkeit kann sich für Anleger auszahlen. Zur Höhe der gezahlten Abfindung beim Stada-Squeeze-Out sind immer noch Klagen ehemaliger Aktionäre anhängig. So ist der aktuelle Stand. Der Hintergrund:... ► Artikel lesen | |
| ELI LILLY | 894,80 | -0,01 % | Goldman Sachs Reaffirms Buy on Eli Lilly and Company (LLY), Citing 25% Growth Outlook | ||
| MERCK & CO | 103,20 | 0,00 % | Insider trades: Merck, Intel, Micron among notable names |
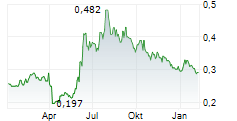
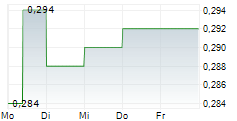
LUYE PHARMA GROUP LTD gehört der Branche Pharma an. Mit einer aktuellen Kursveränderung von 0,000 (0,00 %) liegt der Kurs bei 0,292 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu LUYE PHARMA GROUP LTD finden Sie auf Wallstreet Online.