- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| LYELL IMMUNOPHARMA Aktie jetzt für 0€ handeln | |||||
| 22.12.25 | Lyell Immunopharma pops on recent ASH data, immunotherapy expansion | 4 | Seeking Alpha | ||
| 07.12.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Presents New Clinical Data from Ongoing Trial of Ronde-Cel Showing High Rates of Durable Complete Responses in Patients with Large B-cell Lymphoma at the 67th ASH Annual Meeting and Exposition | 2 | GlobeNewswire (USA) | ||
| 05.12.25 | Lyell Immunopharma, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 13.11.25 | Lyell Immunopharma reports Q3 results | 3 | Seeking Alpha | ||
| 12.11.25 | Lyell Immunopharma, Inc. - 10-Q, Quarterly Report | - | SEC Filings | ||
| 10.11.25 | Lyell Immunopharma buys exclusive global rights to colorectal cancer drug | 1 | Seeking Alpha | ||
| 10.11.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Acquires Exclusive Global Rights to a Next-Generation CAR T-Cell Product Candidate in Clinical Development for Metastatic Colorectal Cancer | 131 | GlobeNewswire (Europe) | LYL273 has demonstrated a 67% overall response rate, an 83% disease control rate, and a manageable safety profile at the highest dose level studied to date in patients with refractory metastatic colorectal... ► Artikel lesen | |
| 10.11.25 | Lyell Immunopharma, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 03.11.25 | Lyell Immunopharma: Vielversprechende Daten zu dualer CAR-T-Zelltherapie bei Lymphomen | 1 | Investing.com Deutsch | ||
| 03.11.25 | Lyell Immunopharma, Inc: Lyell Announces Two Oral Presentations from the Phase 1/2 Clinical Trial of Ronde-Cel for the Treatment of Aggressive Large B-Cell Lymphoma at the 67th ASH Annual Meeting and Exposition | 2 | GlobeNewswire (USA) | ||
| 03.11.25 | Lyell Immunopharma, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 26.09.25 | Lucid Capital Markets initiates Lyell Immunopharma stock with Buy rating | 2 | Investing.com | ||
| 16.09.25 | Lyell Immunopharma, Inc. - 8-K, Current Report | 1 | SEC Filings | ||
| 12.08.25 | Lyell Immunopharma, Inc. - 10-Q, Quarterly Report | 2 | SEC Filings | ||
| 12.08.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Reports Business Highlights and Financial Results for the Second Quarter 2025 | 391 | GlobeNewswire (Europe) | Presented positive new clinical data demonstrating high rates of durable complete responses from the Phase 1/2 trial of LYL314 for the treatment of aggressive large B-cell lymphomaInitiated the PiNACLE... ► Artikel lesen | |
| 12.08.25 | Lyell Immunopharma, Inc. - 8-K, Current Report | 2 | SEC Filings | ||
| 17.06.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Announces Positive New Clinical Data Demonstrating High Rates of Durable Complete Responses from the Phase 1/2 Trial of LYL314 for the Treatment of Aggressive Large B-cell Lymphoma | 217 | GlobeNewswire (Europe) | LYL314 demonstrated robust clinical responses, with an 88% overall response rate and a 72% complete response rate in patients treated in the third- or later-line setting (N = 25)71% of patients with... ► Artikel lesen | |
| 09.06.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Strengthens Clinical and Commercial Capabilities with Key Board and Executive Appointments | 252 | GlobeNewswire (Europe) | Mark J. Bachleda, PharmD, MBA appointed as independent member of the Board of DirectorsDavid Shook, MD appointed as Chief Medical Officer, Mark Meltz, JD as General Counsel and Corporate Secretary... ► Artikel lesen | |
| 13.05.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Reports Business Highlights and Financial Results for the First Quarter 2025 | 306 | GlobeNewswire (Europe) | Presenting new clinical data from Phase 1/2 multi-center clinical trial of LYL314, a next-generation dual-targeting CD19/CD20 CAR T-cell product candidate for the treatment of relapsed and/or refractory... ► Artikel lesen | |
| 15.04.25 | Lyell Immunopharma, Inc: Lyell Immunopharma Receives Regenerative Medicine Advanced Therapy (RMAT) Designation for LYL314 for the Treatment of Relapsed and/or Refractory Large B-Cell Lymphoma | 239 | GlobeNewswire (Europe) | RMAT designation was granted based on promising clinical data from the ongoing Phase 1/2 trial of LYL314 in patients with relapsed and/or refractory large B-cell lymphoma.RMAT designation recognizes... ► Artikel lesen |
1 2 Weiter >>
21 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| MEDIGENE | 0,042 | -8,26 % | Medigene: Zurückziehung - 18.11.2025 | ||
| AMGEN | 325,15 | +0,06 % | Märkte am Morgen: "KI-Realitätscheck", Wolters Kluwer, AMD, Amgen, Walmart, Novo Nordisk | Die Stimmung an den Aktienmärkten ist derzeit sehr, sehr wechselhaft. Das war gestern deutlich zu spüren. Der DAX war bemerkenswert stark in den Handel gestartet, schloss allerdings leicht im Minus.... ► Artikel lesen | |
| BIOGEN | 164,25 | +3,79 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| EDITAS MEDICINE | 1,536 | +0,03 % | Editas Medicine, Inc.: Editas Medicine Nominates EDIT-401, an LDLR-Targeted Medicine, as Lead In Vivo Development Candidate | EDIT-401 achieved ~90% mean LDL-C reduction with single dose in non-human primates EDIT-401 on track for human proof-of-concept data by end of 2026 Strong cash position with operational runway into... ► Artikel lesen | |
| GALAPAGOS NV | 28,120 | -2,97 % | Galapagos NV: Galapagos Announces Board Decision to Initiate Wind-Down of Cell Therapy Activities | Mechelen, Belgium; January 5, 2026, 22:01 CET; Galapagos NV (Euronext & NASDAQ: GLPG) today announced that the works council consultation process regarding the wind-down of cell therapy activities has... ► Artikel lesen | |
| IMMATICS | 7,620 | -6,22 % | Immatics Presents IMA203CD8 PRAME Cell Therapy Data from Ongoing Dose Escalation and Shows Promising Initial Anti-tumor Activity in PRAME-Positive Tumors at ESMO-IO 2025 Congress | IMA203CD8 is a second-generation PRAME cell therapy with enhanced pharmacology in ongoing Phase 1a dose escalationManageable tolerability across all dose levelsEncouraging early clinical anti-tumor... ► Artikel lesen | |
| AUTOLUS THERAPEUTICS | 1,190 | -0,83 % | Autolus Therapeutics plc: Autolus Therapeutics Announces Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | ||
| IGM BIOSCIENCES | 1,080 | 0,00 % | XFRA DELETION OF INSTRUMENTS FROM BOERSE FRANKFURT - 14.08.2025 | The following instruments on Boerse Frankfurt do have their last trading day on 14.08.2025.Die folgenden Instrumente in Boerse Frankfurt haben ihren letzten Handelstag am 14.08.2025.ISIN NameCA87168M1068 SYNEX... ► Artikel lesen | |
| TANGO THERAPEUTICS | 12,530 | +4,85 % | Expert Outlook: Tango Therapeutics Through The Eyes Of 4 Analysts | ||
| TSCAN THERAPEUTICS | 1,010 | +10,94 % | TScan Therapeutics, Inc.: TScan Therapeutics Announces Positive Updated Data from the ALLOHA Phase 1 Heme Trial at the 67th American Society of Hematology Annual Meeting and Exposition | Treatment arm continues to demonstrate favorable relapse-free survival (HR=0.50; p=0.23) and overall survival (HR=0.61; p=0.52) 3/3 (100%) of TSC-101-treated patients who reached two-year follow-up... ► Artikel lesen | |
| PRAXIS PRECISION MEDICINES | 319,57 | +4,56 % | Praxis Precision Medicines, Inc. Announces Inducement Grants Under Nasdaq Listing Rule 5635(c)(4) | ||
| QIAGEN | 43,170 | -0,70 % | JEFFERIES stuft QIAGEN NV auf 'Buy' | NEW YORK (dpa-AFX Analyser) - Das Analysehaus Jefferies hat die Einstufung für Qiagen mit einem Kursziel von 57 US-Dollar auf "Buy" belassen. Umsatz und Ergebnis des vierten Quartals hätten die Erwartungen... ► Artikel lesen | |
| AVIDITY BIOSCIENCES | 72,90 | +0,11 % | Avidity Biosciences, Inc. - 8-K, Current Report | ||
| TARSUS PHARMACEUTICALS | 64,62 | +4,24 % | Tarsus Pharmaceuticals, Inc: Tarsus Reports Third Quarter 2025 Financial Results and Recent Business Achievements | Delivered quarterly XDEMVY® net sales of approximately $119 million, up approximately 147% year-over-year Weekly multi-patient prescribers grew approximately 30% in the third quarter underscoring... ► Artikel lesen | |
| SUMMIT THERAPEUTICS | 14,990 | +8,23 % | H.C. Wainwright reiterates Buy rating on Summit Therapeutics stock |
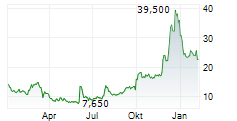
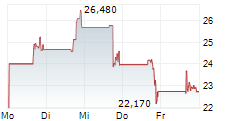
LYELL IMMUNOPHARMA INC gehört der Branche Biotechnologie an. Mit einer aktuellen Kursveränderung von +0,080 (+0,35 %) liegt der Kurs bei 22,720 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu LYELL IMMUNOPHARMA INC finden Sie auf Wallstreet Online.