| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
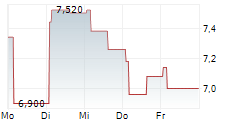
| 6,300 | 6,320 | 10:26 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Mo | MaaT Pharma To Present New Promising Preclinical Data at AACR for MaaT034 Aiming To Improve Patients' Responses to Immunotherapies | 195 | Business Wire | Regulatory News:
MaaT Pharma (EURONEXT: MAAT the "Company"), a clinical-stage biotechnology company and a leader in the development of Microbiome Ecosystem TherapiesTM (MET) dedicated to enhancing... ► Artikel lesen | |
| 27.03. | MaaT Pharma Completes a Capital Increase of €13 Million with Historical Shareholders and Announces 2024 Annual Results | 707 | Business Wire | Positive results from Phase 3 trial for MaaT013 in acute Graft-versus-Host disease (aGvHD); topline results (January 2025) showed a 62% gastrointestinal overall response rate at Day 28 and 1-year... ► Artikel lesen | |
| 19.03. | MaaT Pharma stock adds 7% on DSMB nod | 3 | Investing.com | ||
| 18.03. | MaaT Pharma Announces Positive Outcomes from Final DSMB Meeting for Pivotal Phase 3 Clinical Trial Evaluating MaaT013 in Acute Graft-versus-Host Disease | 247 | Business Wire | The Independent Data Safety and Monitoring Board (DSMB) confirmed the remarkable efficacy results and a positive benefit/risk profile of MaaT013 in this patient population
The Company plans... ► Artikel lesen | |
| 11.03. | MaaT Pharma Receives Positive Opinion from EMA Pediatric Committee on the Pediatric Investigation Plan for MaaT013 | 330 | Business Wire | Positive EMA Pediatric Committee opinion has cleared the investigation clinical plan to evaluate the safety and efficacy of MaaT013 in patients from 6 years old to less than 18 years old with... ► Artikel lesen | |
| 21.01. | MaaT Pharma Announces Positive Second DSMB Review of Ongoing Phase 2b Clinical Trial Evaluating MaaT033 for Patients Receiving Allo-HSCT | 398 | Business Wire | The Independent Data Safety and Monitoring Board (DSMB) has recommended that the trial proceeds as planned without modification. Consistent good safety profile and tolerability for MaaT033... ► Artikel lesen | |
| 08.01. | MaaT Pharma Announces Positive Topline Results from the Pivotal Phase 3 ARES Study Evaluating MaaT013 in acute Graft-versus-Host Disease | 540 | Business Wire | The study met its primary endpoint with a significant gastrointestinal overall response rate at Day 28 of 62% and demonstrates the unprecedented efficacy of MaaT013 as third-line treatment of aGvHD... ► Artikel lesen | |
| MAAT PHARMA Aktie jetzt für 0€ handeln | |||||
| 07.01. | MaaT Pharma: Monthly Information Regarding the Total Number of Voting Rights and Shares Comprising the Share Capital | 324 | Business Wire | Article 223-16 of the General Regulations of the Financial Markets Authority (AMF Autorité des Marchés Financiers)
Regulatory News:
MaaT Pharma (EURONEXT: MAAT the "Company"), a clinical-stage... ► Artikel lesen | |
| 10.12.24 | MaaT Pharma Presented Positive Updated Data on MaaT013 in the Early Access Program at ASH 2024 Annual Meeting | 331 | Business Wire | MaaT Pharma to host a KOL webinar on December 17th, 2024, to discuss data and the unmet medical need in acute Graft-versus-Host Disease (aGvHD) Register here.
Sustained High Response Rates... ► Artikel lesen | |
| 05.12.24 | MaaT Pharma Announces First U.S. Patient Treated at City of Hope Under Single Patient Expanded Access for MaaT013 in Acute Graft-versus-Host Disease | 571 | Business Wire | Regulatory News:
MaaT Pharma (EURONEXT: MAAT the "Company"), a clinical-stage biotechnology company and a leader in the development of Microbiome Ecosystem TherapiesTM (MET) dedicated to enhancing... ► Artikel lesen | |
| 26.11.24 | MaaT Pharma Announces Positive Phase 1b Results, Meeting Primary Endpoint in the Evaluation of MaaT033 in Amyotrophic Lateral Sclerosis (ALS) | 78 | Business Wire | MaaT033 administered for two months confirmed good safety profile and was well tolerated in patients with ALS. Other study endpoints will be analysed in the upcoming months with full data... ► Artikel lesen | |
| 07.11.24 | MaaT Pharma to Present Updates from Early Access Program at the 2024 ASH Annual Meeting Demonstrating Prolonged Long-Term Survival in Patients Receiving MaaT013 in aGvHD | 448 | Business Wire | Efficacy, safety, and long-term follow-up data from 154 patients in the EAP in Europe further reinforce the excellent clinical profile of MaaT013 in GI-aGvHD.
MaaT013 is a safe and effective... ► Artikel lesen | |
| 05.11.24 | MaaT Pharma Provides Third Quarter 2024 Business and Financial Results and Announces Management Changes | 363 | Business Wire | Completion of enrollment in the European Phase 3 clinical trial ARES designed to evaluate efficacy and safety of MaaT013 in the treatment of acute Graft-versus-Host Disease; topline results expected... ► Artikel lesen | |
| 15.10.24 | MaaT Pharma Completes Recruitment of its ARES Phase 3 Trial for MaaT013 to Treat Acute Graft-versus-Host Disease | 289 | Business Wire | Last patient treated in MaaT Pharma's Phase 3 ARES clinical trial
Topline results publication now expected in January 2025
Positive DSMB review of Phase 3 ARES trial announced in... ► Artikel lesen | |
| 19.09.24 | MaaT Pharma Publishes its Half Year 2024 Results and Provides a Business Update | 393 | Business Wire | Positive efficacy and safety data of MaaT013 in aGvHD in the Early Access Program presented at the EBMT 2024 annual meeting with 63% GI-ORR at D28, a 49% one year and 42% 18 months Overall Survival... ► Artikel lesen | |
| 09.09.24 | MaaT Pharma: Monthly Information Regarding the Total Number of Voting Rights and Shares Comprising the Share Capital | 427 | Business Wire | Article 223-16 of the General Regulations of the Financial Markets Authority (AMF Autorité des Marchés Financiers)
Regulatory News:
MaaT Pharma (EURONEXT: MAAT the "Company"), a clinical-stage... ► Artikel lesen | |
| 04.09.24 | MaaT Pharma To Present and Participate in Investor and Medical Conferences in September | 365 | Business Wire | Regulatory News:
MaaT Pharma(EURONEXT: MAAT the "Company"), a clinical-stage biotechnology company and a leader in the development of Microbiome Ecosystem Therapies (MET) dedicated to enhancing... ► Artikel lesen | |
| 09.08.24 | MaaT Pharma: MaaT Pharma: Monthly Information Regarding the Total Number of Voting Rights and Shares Comprising the Share Capital | 537 | Business Wire | Article 223-16 of the General Regulations of the Financial Markets Authority (AMF Autorité des Marchés Financiers)
Regulatory News:
MaaT Pharma (EURONEXT: MAAT the "Company"), a clinical-stage... ► Artikel lesen | |
| 05.07.24 | MaaT Pharma: Monthly Information Regarding the Total Number of Voting Rights and Shares Comprising the Share Capital | 858 | Business Wire | Article 223-16 of the General Regulations of the Financial Markets Authority (AMF Autorité des Marchés Financiers)
Regulatory News:
MaaT Pharma (EURONEXT: MAAT the "Company"), a clinical-stage... ► Artikel lesen | |
| 05.07.24 | MaaT Pharma: Half-year Report and an Increase of the Resources Allocated to the Liquidity Contract With Kepler Cheuvreux | 690 | Business Wire | Regulatory News:
MaaT Pharma (EURONEXT: MAAT the "Company"), a clinical-stage biotechnology company and a leader in the development of Microbiome Ecosystem TherapiesTM (MET) dedicated to enhancing... ► Artikel lesen |
Seite:
1 2 Weiter >>
34 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| INTELLIA THERAPEUTICS | 6,340 | -5,85 % | Intellia Focuses on Pipeline Development Amid Stiff Competition | ||
| PACIFIC BIOSCIENCES OF CALIFORNIA | 1,070 | -8,23 % | PacBio Appoints Jim Gibson As New CFO | WASHINGTON (dpa-AFX) - Life science technology company PacBio, Inc. (PACB) announced Thursday the appointment of Jim Gibson as the company's new Chief Financial Officer, effective as of his... ► Artikel lesen | |
| CENTOGENE | 0,120 | 0,00 % | Centogene NV: CENTOGENE Closes Strategic Transaction with Private Equity Group Charme Capital Partners | CAMBRIDGE, Mass. and ROSTOCK, Germany and BERLIN, March 12, 2025 (GLOBE NEWSWIRE) -- Centogene N.V. (OTC: CNTGF) ("CENTOGENE" or the "Company") today announced it has closed its transaction to sell... ► Artikel lesen | |
| ATAI LIFE SCIENCES | 1,300 | -3,78 % | ATAI Life Sciences Aktie: Bericht über jüngste Finanztrends | Das Biotechnologie-Unternehmen erforscht innovative Substanzen gegen Depressionen und entwickelt neuartige Behandlungsmethoden für psychische Erkrankungen. ATAI Life Sciences treibt die Entwicklung... ► Artikel lesen | |
| BIOMERIEUX | 118,60 | +1,19 % | bioMérieux receives FDA 510(k) Clearance for its VITEK COMPACT PRO, a new ID/AST system to fight against antimicrobial resistance | MARCY L'ÉTOILE, France, March 18, 2025 /PRNewswire/ -- bioMérieux, a world leader in the field of in vitro diagnostics, announces the U.S. Food and Drug Administration... ► Artikel lesen | |
| CYCLACEL | 0,296 | -100,00 % | Cyclacel Pharmaceuticals, Inc. - 10-K, Annual Report | ||
| SYROS PHARMACEUTICALS | 0,108 | -100,00 % | XFRA DELETION OF INSTRUMENTS FROM BOERSE FRANKFURT - 25.03.2025 | The following instruments on Boerse Frankfurt do have their last trading day on 25.03.2025.Die folgenden Instrumente in Boerse Frankfurt haben ihren letzten Handelstag am 25.03.2025.ISIN NameCA05553A1075 THE... ► Artikel lesen | |
| XTL BIOPHARMACEUTICALS | 1,090 | +5,83 % | XTL BIOPHARMACEUTICALS LTD - 6-K, Report of foreign issuer | ||
| GENUS | 21,400 | -1,83 % | Genus - Director/PDMR Shareholding | ||
| ANAPTYSBIO | 17,100 | -2,29 % | AnaptysBio, Inc.: Anaptys Announces Stock Repurchase Plan | SAN DIEGO, March 24, 2025 (GLOBE NEWSWIRE) -- AnaptysBio, Inc. (Nasdaq: ANAB), a clinical-stage biotechnology company focused on delivering innovative immunology therapeutics, today announced that... ► Artikel lesen | |
| ABIONYX PHARMA | 1,178 | -0,51 % | CIC Market Solutions Initiates Buy Coverage of ABIONYX Pharma | Regulatory News:
ABIONYX Pharma (FR0012616852 ABNX PEA PME eligible), next-generation biopharma dedicated to the development of innovative biomedicines based on recombinant apolipoprotein apoA-I... ► Artikel lesen | |
| MEIRAGTX | 5,900 | +0,85 % | MeiraGTx Announces The Lancet Publication of Data Demonstrating the Efficacy of rAAV8.hRKp.AIPL1 for the Treatment of Leber Congenital Amaurosis 4 (LCA4) Retinal Dystrophy | As disclosed in the paper, 4 out of 4 young children with the AIPL1-related retinal dystrophy, LCA4, benefited substantially from unilateral subretinal administration of rAAV8.hRKp.AIPL1 with improved... ► Artikel lesen | |
| METABOLIC EXPLORER | 0,095 | 0,00 % | XFRA DELETION OF INSTRUMENTS FROM BOERSE FRANKFURT - 09.10.2024 | The following instruments on Boerse Frankfurt do have their last trading day on 09.10.2024Die folgenden Instrumente in Boerse Frankfurt haben ihren letzten Handelstag am 09.10.2024ISIN NameAU0000064966 VIRG.MON.... ► Artikel lesen | |
| AKESO | 10,200 | -5,56 % | Akeso, Inc.: Akeso's 2024 Results: Strengthening Global Competitiveness and Transforming the Treatment Landscape with Bispecific Antibodies | Key Highlights:
Global first "immuno + anti-vascular" bispecific antibody approved: ivonescimab for EGFR-TKI resistant nsq-NSCLC. Phase III HARMONi study topline... ► Artikel lesen | |
| ISOFOL MEDICAL | 0,169 | -1,29 % | Isofol Medical AB: Isofol Medical AB (publ) publishes year-end report, January - December 2024 | GOTHENBURG, Sweden, February 19, 2025 - Isofol Medical AB (publ), (Nasdaq Stockholm: ISOFOL), announced today that the company's year-end report for January - December 2024 is now available on the company's... ► Artikel lesen |
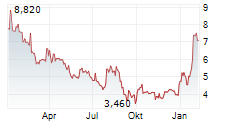
MAAT PHARMA SA gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell -2,52 % bei einem Aktienkurs von 6,180 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und .
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu MAAT PHARMA SA finden Sie auf Wallstreet Online.