| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
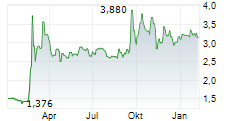
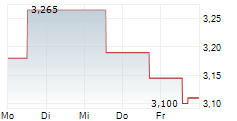
| 3,095 | 3,170 | 13:05 | |
| 3,095 | 3,165 | 06.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| Di | Oryzon obtains grant from Japanese Patent Office for vafidemstat | 1 | thecorner.eu | ||
| 23.12.25 | Oryzon Genomics obtains patent in Japan for 'Combinations of iadademstat for cancer therapy' | 1 | thecorner.eu | ||
| 22.12.25 | Oryzon Genomics, S.A.: ORYZON Expands Global Patent Protection for Iadademstat with Grant Decision in Japan Covering Combinations with PD-1/PD-L1 Inhibitors | 286 | GlobeNewswire (Europe) | MADRID and CAMBRIDGE, Mass., Dec. 22, 2025 (GLOBE NEWSWIRE) -- Oryzon Genomics, S.A. (ISIN Code: ES0167733015, ORY), a clinical-stage biopharmaceutical company and global leader in epigenetics, today... ► Artikel lesen | |
| 15.12.25 | Oryzon to increase capital through cash contributions and with exclusion of pre-emptive subscription rights, for effective amount of up to €125 million | 2 | thecorner.eu | ||
| 15.12.25 | Oryzon Genomics, S.A.: ORYZON Announces the Voting Results of December 2025 Extraordinary General Shareholders' Meeting | 303 | GlobeNewswire (Europe) | 37.8% of voting rights present or representedAll resolutions were approved MADRID and CAMBRIDGE, Mass., Dec. 15, 2025 (GLOBE NEWSWIRE) -- Oryzon Genomics S.A. (ISIN Code: ES0167733015, ORY), a clinical-stage... ► Artikel lesen | |
| ORYZON GENOMICS Aktie jetzt für 0€ handeln | |||||
| 12.12.25 | ORYZON GENOMICS, S.A.: ORYZON announces the voting results from its Extraordinary General Shareholders' Meeting | 1 | CNMV | ||
| 09.12.25 | Oryzon Genomics, S.A.: ORYZON Presents Data for Iadademstat Combinations in AML at the American Society of Hematology (ASH) 67th Annual Meeting | 1 | GlobeNewswire (USA) | ||
| 09.12.25 | ORYZON GENOMICS, S.A.: ORYZON presents encouraging updated data for iadademstat in AML at the American Society of Hematology (ASH) Meeting | 3 | CNMV | ||
| 11.11.25 | Oryzon Genomics - Progression across the pipeline in Q3 | 328 | Edison Investment Research | Oryzon Genomics has reported its Q325 results, a period characterised by tangible progress across its ongoing clinical programmes. Anticipation builds for vafidemstat in borderline personality disorder... ► Artikel lesen | |
| 10.11.25 | ORYZON GENOMICS, S.A.: Call of the Extraordinary General Shareholders' Meeting of Oryzon Genomics, S.A. | 3 | CNMV | ||
| 07.11.25 | Oryzon Genomics S.A. GAAP EPS of $0.01 | 1 | Seeking Alpha | ||
| 07.11.25 | Oryzon Genomics, S.A.: ORYZON Reports Financial Results and Corporate Update for Quarter Ended September 30, 2025 | 204 | GlobeNewswire (Europe) |
The net result as of September 30, 2025 improved by $1.1M compared to September 2024, following termination of a Convertible Bonds financing facility with Nice&Green CNS(Vafidemstat) Received feedback... ► Artikel lesen | |
| 04.11.25 | Oryzon Genomics - Progress for iadademstat across the board | 289 | Edison Investment Research | Oryzon has announced encouraging updates for iadademstat. In acute myeloid leukaemia (AML), positive data was reported for two programmes, including the lead oncology programme, FRIDA. Updated interim... ► Artikel lesen | |
| 03.11.25 | Oryzon Genomics, S.A.: ORYZON Announces First-Patient-In (FPI) in RESTORE Phase Ib Trial of Iadademstat in Sickle Cell Disease | 235 | GlobeNewswire (Europe) | Recently approved by the European Medicines Agency, RESTORE will evaluate iadademstat's ability to raise fetal hemoglobin (HbF) in adult patients with SCDIadademstat has demonstrated a favorable safety... ► Artikel lesen | |
| 30.10.25 | Oryzon Genomics, S.A.: ORYZON to Participate in Upcoming Events in November | 3 | GlobeNewswire (USA) | ||
| 20.10.25 | Oryzon Genomics - Making headway towards Phase III in BPD | 361 | Edison Investment Research | Oryzon Genomics has announced that it has received FDA feedback on its plans for the Phase III programme in borderline personality disorder (BPD) for its lead central nervous system (CNS) drug candidate... ► Artikel lesen | |
| 17.10.25 | Oryzon Genomics: FDA gibt Feedback zu Phase-III-Studienprotokoll für Vafidemstat | 2 | Investing.com Deutsch | ||
| 17.10.25 | FDA provides feedback on Oryzon's vafidemstat BPD trial protocol | 7 | Investing.com | ||
| 17.10.25 | Oryzon Genomics, S.A.: ORYZON Receives Feedback From the FDA in Response to the Submitted Phase III Protocol in Borderline Personality Disorder | 289 | GlobeNewswire (Europe) | Provides clarity on the aspects to incorporateCompany to address them and resubmit revised protocol MADRID, Spain and CAMBRIDGE, Mass., Oct. 17, 2025 (GLOBE NEWSWIRE) -- Oryzon Genomics, S.A. (ISIN... ► Artikel lesen | |
| 01.10.25 | Oryzon Genomics, S.A.: ORYZON Strengthens Patent Portfolio for iadademstat with European Grant Decision Covering Combinations with PD1/PD-L1 Inhibitors | 179 | GlobeNewswire (Europe) | MADRID and CAMBRIDGE, Mass., Oct. 01, 2025 (GLOBE NEWSWIRE) -- Oryzon Genomics, S.A. (ISIN Code: ES0167733015, ORY), a clinical-stage biopharmaceutical company and European leader in epigenetics,... ► Artikel lesen |
1 2 Weiter >>
39 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 90,40 | +0,17 % | Startschuss! BioTech-Sektor profitiert von Rotation! Augen auf bei Evotec, Bayer, Vidac Pharma and BioNTech | Mit positiver Grundstimmung hat die Börse das Jahr 2026 eingeläutet. Dass Renditechancen nicht allein im Technologiesektor liegen, bewies zuletzt der Minen- und Rohstoffbereich, wo etliche Titel Kursverdopplungen... ► Artikel lesen | |
| EVOTEC | 6,148 | +0,79 % | SDAX: Evotec-Aktie schießt zweistellig nach oben | Eine Kaufempfehlung von Berenberg entfacht neuen Optimismus bei Evotec und katapultiert die Biotech-Aktie an die Indexspitze. Die Aktie der Evotec hat am Dienstagmittag ein Kursfeuerwerk gezündet und... ► Artikel lesen | |
| MEDIGENE | 0,042 | -8,26 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 43,170 | -0,70 % | Ionos, Kontron, Lanxess, Qiagen, Redcare Pharmacy, Siltronic, TeamViewer: Neue Aktien-Positionen der Short-Seller | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen | |
| MODERNA | 34,650 | -0,07 % | Arbutus jumps on ruling in patent dispute with Moderna | ||
| PAION | 0,200 | +8,11 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WIRD GEPRUEFT | Fuer folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wird ein Mistrade-Antrag geprueft:ISIN Datum Zeit Volumen Preis Fair Value (AS)DE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 3,986 | +0,61 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| AMGEN | 325,15 | +0,06 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| EPIGENOMICS | 0,870 | 0,00 % | PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025 | DJ PTA-Adhoc: Epigenomics AG: Vorläufiges Jahresergebnis zum 31. Dezember 2025
Veröffentlichung von Insiderinformationen gemäß Artikel 17 MAR
Epigenomics AG: Vorläufiges Jahresergebnis zum... ► Artikel lesen | |
| NOVAVAX | 6,910 | -0,80 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| STRYKER | 302,10 | -0,33 % | Stryker Corporation: Stryker declares an $0.88 per share quarterly dividend | Portage, Michigan, Feb. 04, 2026 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) announced that its Board of Directors has declared a quarterly dividend of $0.88 per share payable April 30, 2026, to shareholders... ► Artikel lesen | |
| BIOGEN | 164,25 | +3,79 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,420 | -1,22 % | PTA-news: Biofrontera AG: Biofrontera berichtet über die Ergebnisse der ersten neun Monate des Jahres 2025 | DJ PTA-News: Biofrontera AG: BIOFRONTERA BERICHTET ÜBER DIE ERGEBNISSE DER ERSTEN NEUN MONATE DES JAHRES 2025
Unternehmensmitteilung für den Kapitalmarkt
Biofrontera AG: BIOFRONTERA... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,910 | 0,00 % | HEIDELBERG PHARMA AG zeigt klare Stärke im Trend | ||
| ILLUMINA | 101,04 | -0,18 % | Illumina-Aktie -10%: Kann man hier günstig einsteigen? | Mit einem Kursverlust von -10% war die Illumina-Aktie am Freitag der große Verlierer im S&P 500. Der Hersteller von Gensequenzierungsmaschinen setzte damit seinen Abwärtstrend der letzten Tage fort.... ► Artikel lesen |
ORYZON GENOMICS SA gehört der Branche Biotechnologie an. Die relative Kursveränderung beträgt aktuell -0,64 % bei einem Aktienkurs von 3,110 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu ORYZON GENOMICS SA finden Sie auf Wallstreet Online.