| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
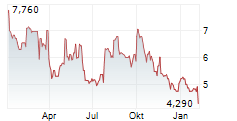
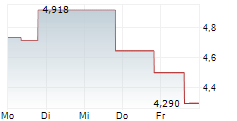
| 4,270 | 4,378 | 07.02. | |
| 4,304 | 4,346 | 06.02. |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 29.01. | OSE Immunotherapeutics Selects Chronic Pouchitis and Hidradenitis Suppurativa as New Key Indications for Lusvertikimab | 76 | GlobeNewswire (Europe) | OSE Immunotherapeutics Selects Chronic Pouchitis and Hidradenitis Suppurativa as New Key Indications for Lusvertikimab Development built on strong IL-7R biological rationaleChronic Pouchitis offers... ► Artikel lesen | |
| 21.01. | OSE Immunotherapeutics Welcomes FDA Orphan Drug Designation Granted to Pegrizeprument (VEL-101) | 305 | GlobeNewswire (Europe) | OSE Immunotherapeutics Welcomes FDA Orphan Drug Designation Granted to Pegrizeprument (VEL-101) Nantes, France, January 21, 2026 - 6pm CET - OSE Immunotherapeutics SA (ISIN: FR0012127173; Mnemo: OSE)... ► Artikel lesen | |
| 20.01. | OSE Immunotherapeutics: Half-Year Report on Liquidity Contract with Invest Securities | 11 | GlobeNewswire (USA) | ||
| 09.01. | OSE Immunotherapeutics - Renewed focus on advancing core assets | 355 | Edison Investment Research | We revisit our investment case for OSE Immunotherapeutics following the recently presented strategic plans for 2026-28, reflecting a more streamlined focus on advancing its core clinical assets, Tedopi... ► Artikel lesen | |
| 09.12.25 | OSE Immunotherapeutics - Strategic plan presented for 2026-2028 | 347 | Edison Investment Research | OSE has announced its strategic plan for the next three years, which is designed to create multiple near-term catalysts. Importantly, it aims to extract maximum value from its lead proprietary candidates... ► Artikel lesen | |
| 09.12.25 | OSE Immunotherapeutics Unveils 2026-2028 Strategic Plan With Four Opportunities for Value Creation | 6 | GlobeNewswire (USA) | ||
| 08.12.25 | AbbVie-Deal belastet: Aktie von Ose Immunotherapeutics gibt nach | 22 | Investing.com Deutsch | ||
| OSE IMMUNOTHERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 08.12.25 | OSE Immunotherapeutics Announces Strategic Amendment to AbbVie's Partnership on ABBV-230 Development | 152 | GlobeNewswire (Europe) | OSE Immunotherapeutics Announces Strategic Amendment to AbbVie's Partnership on ABBV-230 Development Strategic Amendment Enhances OSE Immunotherapeutics' Role in ABBV-230 Development While Preserving... ► Artikel lesen | |
| 17.11.25 | OSE Immunotherapeutics Announces Positive Recommendation from Independent Data Monitoring Committee (IDMC) to Continue Pivotal Phase 3 ARTEMIA Trial Evaluating Tedopi in Non-Small Cell Lung Cancer | 324 | GlobeNewswire (Europe) | OSE Immunotherapeutics Announces Positive Recommendation from Independent Data Monitoring Committee (IDMC) to Continue Pivotal Phase 3 ARTEMIA Trial Evaluating Tedopi® in Non-Small Cell Lung Cancer... ► Artikel lesen | |
| 07.11.25 | OSE Immunotherapeutics Presents a Poster on OSE-Cytomask, a Novel "Cis-Demasking" Cytokine Technology to Increase Therapeutic Index of Immunocytokines | 1 | GlobeNewswire (USA) | ||
| 20.10.25 | OSE Immunotherapeutics - Refocusing for the next phase | 344 | Edison Investment Research | OSE Immunotherapeutics' H125 results, the first since the board restructuring, reflected normalised operations typical of a clinical-stage biotech, following H124's exceptional licensing income. The... ► Artikel lesen | |
| 15.10.25 | OSE Immunotherapeutics Reports First Half 2025 Financial Results | 451 | GlobeNewswire (Europe) | OSE Immunotherapeutics Reports First Half 2025 Financial Results NANTES, France, October 15, 2025 - 7:00pm CET - OSE Immunotherapeutics SA (ISIN: FR0012127173; Mnemo: OSE), today reported its consolidated... ► Artikel lesen | |
| 03.10.25 | OSE ImmunoTherapeutics replaces CEO Nicolas Poirier, appoints Marc Le Bozec as interim CEO | 3 | Seeking Alpha | ||
| 03.10.25 | OSE Immunotherapeutics Names Marc Le Bozec Interim CEO, Effective Immediately | 1 | RTTNews | ||
| 03.10.25 | OSE Immunotherapeutics appoints Marc Le Bozec as interim CEO | 10 | Investing.com | ||
| 03.10.25 | OSE Immunotherapeutics beruft Marc Le Bozec zum Interims-CEO | 2 | Investing.com Deutsch | ||
| 03.10.25 | OSE Immunotherapeutics: Appointment of Marc Le Bozec as Interim CEO | 167 | GlobeNewswire (Europe) | Appointment of Marc Le Bozec as Interim CEO Nantes, October 3, 2025, 8:00 a.m. CET - OSE Immunotherapeutics (ISIN: FR0012127173; Mnemonic: OSE) announces the termination of the position as chief... ► Artikel lesen | |
| 30.09.25 | OSE Immunotherapeutics: Full renewal of the Board of Directors Election of Dr. Markus Cappel as Chairman of the Board of Directors | 171 | GlobeNewswire (Europe) | Full renewal of the Board of DirectorsElection of Dr. Markus Cappel as Chairman of the Board of Directors Nantes, September 30, 2025, 7:30 p.m. CET - OSE Immunotherapeutics (ISIN: FR0012127173; Mnemonic:... ► Artikel lesen | |
| 26.09.25 | OSE Immunotherapeutics - Updated cash position; new collaboration | 432 | Edison Investment Research | While OSE Immunotherapeutics' H125 results were previously due to be presented in September, they are now scheduled for 15 October 2025 to allow for the AGM (30 September 2025) to take place ahead of... ► Artikel lesen | |
| 26.09.25 | Verzögerung bei AbbVie-Partnerschaft belastet Aktie von Ose Immunotherapeutics | 16 | Investing.com Deutsch |
1 2 3 Weiter >>
54 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 90,40 | +0,17 % | Startschuss! BioTech-Sektor profitiert von Rotation! Augen auf bei Evotec, Bayer, Vidac Pharma and BioNTech | Mit positiver Grundstimmung hat die Börse das Jahr 2026 eingeläutet. Dass Renditechancen nicht allein im Technologiesektor liegen, bewies zuletzt der Minen- und Rohstoffbereich, wo etliche Titel Kursverdopplungen... ► Artikel lesen | |
| EVOTEC | 6,148 | +0,79 % | Zink-Boom, Turnaround, Biotech-Wachstum: So profitieren Sie mit Pasinex Resources, Puma und Evotec | In volatilen Märkten suchen Anleger nach außergewöhnlichen Chancen. Drei Unternehmen stechen dabei heraus. Ein Rohstoffunternehmen mit außergewöhnlichen Zink-Projekten, ein Sportartikelhersteller im... ► Artikel lesen | |
| BB BIOTECH | 50,60 | -0,78 % | BB Biotech: Gewinnsprung 2025 und höhere Dividende geplant | Die BB Biotech AG hat für das Geschäftsjahr 2025 vorläufig einen Nettogewinn von 578 Millionen CHF ausgewiesen, nach 76 Millionen CHF im Vorjahr. Die Ergebnisse der Biotech-Beteiligungsgesellschaft... ► Artikel lesen | |
| MEDIGENE | 0,042 | -8,26 % | Medigene: Zurückziehung - 18.11.2025 | ||
| QIAGEN | 43,170 | -0,70 % | Diagnostikkonzern Qiagen überrascht im Schlussquartal - Weiteres Wachstum 2026 | VENLO/HILDEN (dpa-AFX) - Qiagen hat 2025 von einer anziehenden Nachfrage nach seinen Kernprodukten profitiert. Dabei lief das Schlussquartal besser als vom Labordienstleister und Diagnostikkonzern... ► Artikel lesen | |
| MODERNA | 34,650 | -0,07 % | Arbutus jumps on ruling in patent dispute with Moderna | ||
| PAION | 0,200 | +8,11 % | XFRA MISTRADE ANTRAG IN ISIN DE000A3E5EG5 WIRD GEPRUEFT | Fuer folgende Geschaefte in der ISIN DE000A3E5EG5, Wertpapier-Name: PAION AG INH O.N., wird ein Mistrade-Antrag geprueft:ISIN Datum Zeit Volumen Preis Fair Value (AS)DE000A3E5EG5 02.02.2026 08:13:40 300 0... ► Artikel lesen | |
| VALNEVA | 3,986 | +0,61 % | Valneva and Instituto Butantan Announce Initiation of a Pilot Vaccination Campaign in Brazil with Single-Shot Chikungunya Vaccine IXCHIQ | Lyon (France), Sao Paulo, (Brazil), February 3, 2026 -Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and Instituto Butantan, one of the world's largest biomedical research... ► Artikel lesen | |
| AMGEN | 325,15 | +0,06 % | Sanofi und Amgen: Droht diesem Ansatz das Aus? AKTIONÄR-Tipp profitiert! | Viele große Pharma- und Biotech-Firmen sind auf der Suche nach neuen Behandlungsansätzen für die Atopische Dermatitis (Neurodermitis). So auch die großen Branchenvertreter Amgen und Sanofi, die mit... ► Artikel lesen | |
| NOVAVAX | 6,910 | -0,80 % | Novavax Licenses Matrix-M Adjuvant To Pfizer For Upfront Payment Of $30 Mln | NEW YORK CITY (dpa-AFX) - Novavax, Inc. (NVAX) announced Tuesday that it has entered into a license agreement with Pfizer, Inc. (PFE) for use of Novavax's Matrix-M adjuvant. Under the terms... ► Artikel lesen | |
| STRYKER | 302,10 | -0,33 % | Stryker Corporation: Stryker declares an $0.88 per share quarterly dividend | Portage, Michigan, Feb. 04, 2026 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) announced that its Board of Directors has declared a quarterly dividend of $0.88 per share payable April 30, 2026, to shareholders... ► Artikel lesen | |
| BIOGEN | 164,25 | +3,79 % | Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen | |
| BIOFRONTERA | 2,420 | -1,22 % | PTA-news: Biofrontera AG: Biofrontera berichtet über die Ergebnisse der ersten neun Monate des Jahres 2025 | DJ PTA-News: Biofrontera AG: BIOFRONTERA BERICHTET ÜBER DIE ERGEBNISSE DER ERSTEN NEUN MONATE DES JAHRES 2025
Unternehmensmitteilung für den Kapitalmarkt
Biofrontera AG: BIOFRONTERA... ► Artikel lesen | |
| HEIDELBERG PHARMA | 2,910 | 0,00 % | HEIDELBERG PHARMA AG zeigt klare Stärke im Trend | ||
| ILLUMINA | 101,04 | -0,18 % | Illumina-Aktie -10%: Kann man hier günstig einsteigen? | Mit einem Kursverlust von -10% war die Illumina-Aktie am Freitag der große Verlierer im S&P 500. Der Hersteller von Gensequenzierungsmaschinen setzte damit seinen Abwärtstrend der letzten Tage fort.... ► Artikel lesen |
Das Unternehmen OSE IMMUNOTHERAPEUTICS SA kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell -0,79 % bei einem Aktienkurs von 4,292 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu OSE IMMUNOTHERAPEUTICS SA finden Sie auf Wallstreet Online.