| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
| 47,000 | 47,200 | 18:32 | |
| 47,000 | 47,200 | 18:34 |
- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 18:10 | Sanofi and Regeneron's high-flying Dupixent on course for an 'inflection year' in COPD | 2 | FiercePharma | ||
| 17:46 | Sanofi stays course amid tariff turmoil | 2 | BioPharma Dive | ||
| 16:06 | French Drugmaker Sanofi Clocks 20% Q1 Profit Growth, Forecasts Strong Rebound In Annual Profit | 5 | Benzinga.com | ||
| 15:30 | Sanofi proposes annual dividend of €3.92, a hike of 4.26% from prior dividend | 8 | Seeking Alpha | ||
| 15:15 | Pharma Giant Roche Tops First-Quarter Views; Sanofi's Sales Come In Mixed | 3 | Investor's Business Daily | ||
| 14:42 | EU pharma majors Sanofi, Roche trade flat after reiterated guidance | 3 | Seeking Alpha | ||
| 13:35 | Sanofi Q1 Earnings Top Estimates, Dupixent Drives Sales Growth | 2 | Zacks | ||
| 13:27 | MIDDAY BRIEFING - Unternehmen und Märkte: DELIVERY HERO, EVOTEC, PFISTERER, AIR LIQUIDE, VOSSLOH, HUYNDAI MOTOR, LG ELECTRONICS, KÜHNE + NAGEL , NESTLE, NOKIA, RELX, RENAULT, SANOFI, STMICROELECTRONICS, UNILEVER | 712 | Dow Jones News | DJ MIDDAY BRIEFING - Unternehmen und Märkte
Der Markt-Überblick am Mittag, zusammengestellt von Dow Jones Newswires:
+++++ FEIERTAGSHINWEIS +++++
FREITAG: An der Börse in Sydney... ► Artikel lesen | |
| SANOFI SA ADR Aktie jetzt für 0€ handeln | |||||
| 13:02 | Sanofi setzt auf Innovationen trotz Marktunsicherheiten | 10 | IT BOLTWISE | ||
| 12:02 | Dividenden-Garant Sanofi gelingt Überraschung - Aktie fällt trotzdem | 304 | Der Aktionär | Der Pharmakonzern Sanofi ist unerwartet stark in das Jahr gestartet. Die Franzosen konnten sowohl beim Umsatz als auch beim Gewinn die Erwartungen des Marktes übertreffen. Das Management um Paul Hudson... ► Artikel lesen | |
| 11:26 | Sanofi's oral TNF inhibitor misses mark in phase 2 psoriasis trial, prompting focus on combos | 10 | FierceBiotech | ||
| 11:26 | ROUNDUP: Sanofi sieht sich nach unerwartet starkem Jahresstart auf Kurs | 142 | dpa-AFX | PARIS (dpa-AFX) - Für den Arzneimittelhersteller Sanofi hat das Jahr unerwartet stark begonnen. Die Franzosen konnten vor allem dank einer guten Nachfrage nach ihrem Kassenschlager Dupixent und einigen... ► Artikel lesen | |
| 09:10 | Pharmakonzern Sanofi sieht sich nach Zuwächsen auf Kurs | 14 | Reuters Deutschland | ||
| 08:39 | Sanofi bekräftigt Ausblick nach starkem Erstquartal | 260 | Dow Jones News | DJ Sanofi bekräftigt Ausblick nach starkem Erstquartal
Von Helena Smolak
DOW JONES--Der Pharmakonzern Sanofi hat im ersten Quartal Umsatz und operativen Gewinn stärker erhöht als erwartet.... ► Artikel lesen | |
| 08:06 | Sanofi Q1 Results Climb; Confirms FY25 Outlook | 201 | AFX News | PARIS (dpa-AFX) - French drug major Sanofi (SNY)' reported Thursday higher profit in its first quarter, with growth in net sales. Further, the company confirmed its fiscal 2025 outlook.In the... ► Artikel lesen | |
| 07:47 | Sanofi kommt zum Jahresstart überraschend gut in Tritt | 11 | cash | ||
| 07:42 | Sanofi beats top-line and bottom-line estimates; reaffirms FY25 outlook | 9 | Seeking Alpha | ||
| 07:36 | Sanofi: strong Q1 performance and 2025 guidance confirmed | 67 | GlobeNewswire (Europe) | Sanofi: strong Q1 performance and 2025 guidance confirmed Paris, April 24, 2025 Q1 sales growth of 9.7% at CER1 and business EPS2 of €1.79 Pharma launches reached sales of €0.8 billion, up 43.8%, driven... ► Artikel lesen | |
| 07:36 | Innate Pharma SA: Innate Pharma Announces €15M Investment by Sanofi | 183 | Business Wire | Investment is in addition to the ongoing partnership as shared previously, including continuing the development of BCMA targeting ANKET program in autoimmune indications, and more
Regulatory... ► Artikel lesen | |
| 07:35 | Pharmakonzern Sanofi kommt zum Jahresstart überraschend gut in Tritt | 157 | dpa-AFX | PARIS (dpa-AFX) - Der Arzneimittelhersteller Sanofi ist unerwartet stark in das Jahr gestartet. Der Umsatz sei im Zeitraum von Januar bis März im Vergleich zum Vorjahr um 10,8 Prozent auf 9,9 Milliarden... ► Artikel lesen |
Seite:
1 2 3 4 5 Weiter >>
678 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BAYER | 22,480 | +1,70 % | Bayer Aktie: Ist das die Lösung im Glyphosat-Streit? | © Foto: FinanznachrichtenDie Bayer AG steht vor einer entscheidenden Weggabelung. Mittendrin in den ganzen Glyphosat-Klagen, hohem Kostendruck und einer angeschlagenen Aktie sucht der DAX-Konzern nach... ► Artikel lesen | |
| NOVO NORDISK | 54,96 | +1,33 % | Novo Nordisk-Aktie: Panik am Markt! | News von Trading-Treff.de Für die Aktie von Novo Nordisk sieht es nach einer absoluten Abwärtsfahrt aus. Mit weiteren -7,4 % ist die Aktie am Donnerstag auf einem extrem schwachen Niveau in das Osterwochenende... ► Artikel lesen | |
| PFIZER | 20,020 | +1,31 % | Pfizer Aktie: Abrysvo voraus! | Experten empfehlen Pfizers RSV-Impfstoff für jüngere Risikogruppen. Potenzieller Markt wächst deutlich - Konkurrenz bleibt stark. Gute Nachrichten für Pfizer aus den USA? Es sieht ganz danach aus. Der... ► Artikel lesen | |
| NOVARTIS | 98,32 | +1,35 % | Novartis: Das Allzeithoch ist in Schlagdistanz! | ||
| MERCK KGAA | 119,75 | -0,70 % | Aktienmarkt: Kurs der Aktie von Merck im Plus (69,1413 €) | Im Plus liegt aktuell die Merck-Aktie . Zuletzt zahlten Investoren für das Papier 78,55 US-Dollar. Ein Preisanstieg von 2,73 Prozent steht gegenwärtig für das Wertpapier von Merck zu Buche. Die Aktie... ► Artikel lesen | |
| GILEAD SCIENCES | 94,37 | +0,52 % | Interessante Einstiege finden - BioNTech, BioNxt Solutions, Gilead Sciences, Merck & Co. | Die aggressive Zollpolitik der US-Administration unter US-Präsident Trump hat zu Umschichtungsprozessen bei den Anlegern geführt. So zogen europäische ETF-Anleger über eine Milliarde Euro aus US-Aktien-ETFs... ► Artikel lesen | |
| AURORA CANNABIS | 4,075 | +4,22 % | Aurora Cannabis Aktie: Ein neuer Lichtblick! | Der kanadische Cannabisproduzent positioniert sich durch internationale Markterschließung, technologische Innovation und solide Finanzlage für langfristiges Wachstum. Aurora Cannabis zieht weiterhin... ► Artikel lesen | |
| SANOFI | 93,33 | -0,07 % | Aktienmarkt: Kurs der Sanofi SA-Aktie im Minus (89,69 €) | Die Sanofi SA-Aktie notiert heute etwas leichter. Das Papier kostete zuletzt 89,69 Euro. Die Aktie von Sanofi SA verzeichnet derzeit einen Abschlag von 0,80 Prozent. Sie hat sich um 72 Cent gegenüber... ► Artikel lesen | |
| GSK | 16,425 | +1,33 % | GSK erhält in den USA Empfehlungen für Meningitis- und RSV-Impfstoffe | DJ GSK erhält in den USA Empfehlungen für Meningitis- und RSV-Impfstoffe
Von Cristina Gallardo
DOW JONES--Der britische Pharmakonzern GSK hat von einem Beratungsgremium des US-Gesundheitsministeriums... ► Artikel lesen | |
| ABBVIE | 159,20 | +1,79 % | ABBVIE mit Prognosesenkung | Der Biopharmakonzern rechnet mit Zusatzkosten in Höhe von 248 Mio. $ im Zusammenhang mit Übernahmen, darunter Meilensteinzahlungen sowie F&E-Aufwendungen. Hintergrund ist der strategische Fokus auf... ► Artikel lesen | |
| ROCHE | 280,25 | +1,82 % | JPMORGAN stuft ROCHE HOLDINGS AG auf 'Underweight' | NEW YORK (dpa-AFX Analyser) - Die US-Bank JPMorgan hat die Einstufung für Roche auf "Underweight" mit einem Kursziel von 230 Franken belassen. Analyst Richard Vosser bezog sich am Donnerstag auf Studiendaten... ► Artikel lesen | |
| CANOPY GROWTH | 1,254 | +16,11 % | Canopy Growth Aktie: Rechtsstreit und Finanzprobleme | Canopy Growth sieht sich mit einer Sammelklage konfrontiert und meldet rückläufige Umsätze. Die geplante Kapitalerhöhung könnte die Aktie weiter belasten. Klagewelle rollt anAnzeigeSollten Anleger sofort... ► Artikel lesen | |
| STADA ARZNEIMITTEL | - | - | MIDDAY BRIEFING - Unternehmen und Märkte: CONTINENTAL, DAIMLER TRUCK, PORSCHE, HUGO BOSS, AUMANN, BAYWA, STADA, BYD, OMV, STELLANTIS | DJ MIDDAY BRIEFING - Unternehmen und Märkte
Der Markt-Überblick am Mittag, zusammengestellt von Dow Jones Newswires:
+++++ AKTIENMÄRKTE (13:18) +++++
zuletzt +/-... ► Artikel lesen | |
| ELI LILLY | 746,80 | +3,19 % | BREAKING: Eli Lilly hebt ab - Daten zu Anti-Fett-Pille - Novo Nordisk unter Druck | Der US-Pharmakonzern Eli Lilly hat die mit Spannung erwarteten Phase-3-Studiendaten (ACHIEVE-1) zu seinem oral verfügbaren Adipositas-Wirkstoffkandidaten Orforglipron veröffentlicht. Die Daten finden... ► Artikel lesen | |
| MERCK & CO | 69,10 | -0,43 % | Merck 'willing to work' with Trump administration on price gap between US, international medicines |
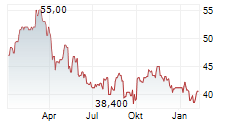
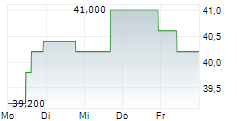
Das Unternehmen SANOFI SA ADR kann der Branche Pharma zugeordnet werden. Mit einer aktuellen Kursveränderung von -1,800 (-3,83 %) liegt der Kurs bei 45,200 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und .
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu SANOFI SA ADR finden Sie auf Wallstreet Online.