- Alle
- Pressemitteilungen
- Empfehlungen
- Chartanalysen
- Berichte
| Zeit | Aktuelle Nachrichten Sprache:
Alle DE EN | Leser | Medien | ||
|---|---|---|---|---|---|
| 11.04. | H.C. Wainwright maintains Unicycive Buy rating, $7.50 target | 3 | Investing.com | ||
| 10.04. | Benchmark maintains $3 target on Unicycive Therapeutics stock | 2 | Investing.com | ||
| 10.04. | Unicycive Therapeutics, Inc.: Unicycive Presents New Patient-Level Data Underscoring Challenges Faced with Current Phosphate Binders and Highlighting the Potential of Oxylanthanum Carbonate to Address Barriers to Adherence for Patients with ... | 4 | GlobeNewswire (USA) | ||
| 01.04. | Unicycive Therapeutics, Inc. - S-8, Securities to be offered to employees in employee benefit plans | 1 | SEC Filings | ||
| 31.03. | Unicycive Therapeutics GAAP EPS of -$0.56 | 2 | Seeking Alpha | ||
| UNICYCIVE THERAPEUTICS Aktie jetzt für 0€ handeln | |||||
| 31.03. | Unicycive Therapeutics, Inc. - 10-K, Annual Report | 2 | SEC Filings | ||
| 31.03. | Unicycive Therapeutics, Inc. Loss At -$31.41 Mln In Full Year | 1 | RTTNews | ||
| 28.01. | Unicycive beleuchtet Herausforderungen im Phosphatmanagement | 3 | Investing.com Deutsch | ||
| 28.01. | Unicycive highlights challenges in phosphate management | 1 | Investing.com | ||
| 28.01. | Unicycive Therapeutics, Inc.: Unicycive Therapeutics Announces the Publication of Patient Perspectives on Phosphate Management in the Journal of Nephrological Science | 1 | GlobeNewswire (USA) | ||
| 07.01. | Unicycive Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 07.01. | Unicycive reports positive phase 1 results for kidney drug | 2 | Investing.com | ||
| 07.01. | Unicycive berichtet über positive Phase-1-Ergebnisse für Nierenmedikament | 2 | Investing.com Deutsch | ||
| 07.01. | Unicycive Therapeutics, Inc.: Unicycive Therapeutics Announces Publication of Positive Oxylanthanum Carbonate (OLC) Dose Escalation Data in Clinical and Translational Science | 1 | GlobeNewswire (USA) | ||
| 13.11.24 | Unicycive Therapeutics GAAP EPS of -$0.05 | 4 | Seeking Alpha | ||
| 13.11.24 | Unicycive Therapeutics, Inc.: Unicycive Announces Third Quarter 2024 Financial Results and Provides Business Update | 185 | GlobeNewswire (Europe) | - OLC New Drug Application (NDA) Accepted by the FDA with a PDUFA Target Action Date of June 28, 2025- - Commercial Planning in Progress for 2025 Launch - - Late Breaker Poster Presentation... ► Artikel lesen | |
| 13.11.24 | Unicycive Therapeutics, Inc. - 8-K, Current Report | - | SEC Filings | ||
| 13.11.24 | Unicycive Therapeutics, Inc. - 10-Q, Quarterly Report | 1 | SEC Filings | ||
| 11.11.24 | Unicycive eyes June FDA verdict for hyperphosphataemia drug | 1 | pharmaphorum | ||
| 11.11.24 | Unicycive Says FDA Accepts NDA For Oxylanthanum Carbonate To Treat Patients With CKD On Dialysis | 3 | RTTNews |
Seite:
1 2 Weiter >>
31 Nachrichten in den letzten 12 Monaten
| Unternehmen / Aktien | Aktienkurs | % | Top-Nachrichten | ||
|---|---|---|---|---|---|
| BIONTECH | 102,20 | +0,79 % | 100-Prozent-Chance: Jetzt bei BioNTech-Rivale Moderna zugreifen? | © Foto: Ian Hutchinson auf UnsplashHinter den beiden Biotech-Schmieden BioNTech und Moderna und ihren Aktien liegen bewegte Jahre. Ließen die Forschungsdurchbrüche der beiden Unternehmen im Bereich... ► Artikel lesen | |
| EVOTEC | 7,344 | +1,75 % | EQS-News: Evotec SE stellt neue Strategie vor und gibt Ausblick für 2025 gestützt durch starkes Ergebnis im 4. Quartal 2024 | EQS-News: Evotec SE
/ Schlagwort(e): Jahresbericht
Evotec SE stellt neue Strategie vor und gibt Ausblick für 2025 gestützt durch starkes Ergebnis im 4. Quartal 2024
17.04.2025... ► Artikel lesen | |
| MEDIGENE | 0,150 | -15,49 % | PTA-AFR: Medigene AG: Vorabbekanntmachung über die Veröffentlichung von Finanzberichten gemäß §§ 114-117 WpHG | DJ PTA-AFR: Medigene AG: Vorabbekanntmachung über die Veröffentlichung von Finanzberichten gemäß §§ 114-117 WpHG
Vorabbekanntmachung Finanzberichte gemäß ---- 114-117 WpHG
Medigene AG: Vorabbekanntmachung... ► Artikel lesen | |
| BB BIOTECH | 30,300 | +0,83 % | BB Biotech Aktie: Vor Quartalszahlen unter Druck | BB Biotech verzeichnet deutliche Kursverluste, doch Analysten halten an positiven Erwartungen fest. Die anstehenden Quartalszahlen könnten neue Impulse bringen. Die BB Biotech-Aktie zeigt sich vor der... ► Artikel lesen | |
| 4SC | 2,680 | -0,37 % | 4SC: Tage der Entscheidung | Die Biotechfirma 4SC (DE000A3E5C40) steckt mitten in einem Nervenkrimi. Nach Jahren der Entwicklung ihres HDAC-Inhibitors Resminostat (Kinselby) zur Behandlung des kutanen T-Zell-Lymphoms (CTCL) wartet... ► Artikel lesen | |
| CRISPR THERAPEUTICS | 34,200 | 0,00 % | Crispr Therapeutics Aktie: Verliert ihren Kurs? | Der Austritt des operativen Geschäftsführers beim Gentherapie-Unternehmen beunruhigt Investoren und wirft Fragen zur Projektkontinuität im wettbewerbsintensiven Biotech-Sektor auf. Die Finanzmärkte... ► Artikel lesen | |
| PALATIN TECHNOLOGIES | 0,188 | +8,94 % | Palatin's Phase 2 Obesity Study Shows Encouraging Appetite Suppression Data, Stock Up In Pre-Market | WASHINGTON (dpa-AFX) - Palatin Technologies, Inc. (PTN), Thursday announced results from its BMT-801 Phase 2 obesity study, which evaluated the co-administration of melanocortin 4 receptor or... ► Artikel lesen | |
| BIOXXMED | 0,412 | +0,49 % | XETR DELETION OF INSTRUMENTS FROM XETRA - 31.03.2025 | The following instruments on Xetra do have their last trading day on 31.03.2025.Die folgenden Instrumente auf Xetra haben ihren letzten Handelstag am 31.03.2025.ISIN NameDE000A4BGGE4 bioXXmed AG ► Artikel lesen | |
| VIVOSIM LABS | 1,930 | -100,00 % | VivoSim Labs, Inc.: VivoSim Announces Emergence from Stealth Mode To Provide Technologies for FDA Turn Away from Animal Models, $10B+ Market | SAN DIEGO, April 24, 2025 (GLOBE NEWSWIRE) -- VivoSim Labs, Inc. (Nasdaq: VIVS) (the "Company") announced that it has emerged from stealth mode to dramatically impact drug discovery and development.... ► Artikel lesen | |
| OCUGEN | 0,656 | +3,63 % | Ocugen Aktie: Leichte Erholung nach schwierigem Jahr | Trotz langfristiger Verluste halten Analysten an positiven Bewertungen für Ocugen fest. Die Gentherapie-Pipeline bleibt der zentrale Hoffnungsträger. Die Ocugen-Aktie zeigt heute eine leichte Erholung... ► Artikel lesen | |
| BIONXT SOLUTIONS | 0,306 | +4,08 % | Interessante Einstiege finden - BioNTech, BioNxt Solutions, Gilead Sciences, Merck & Co. | Die aggressive Zollpolitik der US-Administration unter US-Präsident Trump hat zu Umschichtungsprozessen bei den Anlegern geführt. So zogen europäische ETF-Anleger über eine Milliarde Euro aus US-Aktien-ETFs... ► Artikel lesen | |
| BAVARIAN NORDIC | 20,420 | +1,09 % | Bavarian Nordic Aktie: Zweifel unbegründet? | Trotz heutigem Plus verzeichnet die Bavarian Nordic Aktie erhebliche Verluste seit Jahresbeginn. Technische Indikatoren deuten auf weitere Schwäche hin. Blutbad im LangfristchartAnzeigeSollten Anleger... ► Artikel lesen | |
| INFLARX | 1,161 | +9,12 % | InflaRx N.V. - 6-K, Report of foreign issuer | ||
| SIRONA BIOCHEM | 0,037 | -100,00 % | Sirona Biochem unterzeichnet strategische Beteiligungsvereinbarung und gründet neues Joint Venture mit Promura GmbH | Vancouver, British Columbia - 22. April 2025 / IRW-Press / Sirona Biochem Corp. (TSX-V: SBM) (FWB: ZSB) (OTC:
SRBCF) ("Sirona"), ein Biotechnologieunternehmen, das auf kosmetische Inhaltsstoffe... ► Artikel lesen | |
| VIKING THERAPEUTICS | 22,480 | -1,30 % | Viking Therapeutics Aktie: Wichtige Trends beobachtet | Biotech-Aktie mit starkem Jahresminus, doch Experten sehen hohes Potenzial. Entscheidende Quartalszahlen stehen bevor. Die Viking Therapeutics-Aktie zeigt sich zum Wochenstart mit leichten Gewinnen:... ► Artikel lesen |
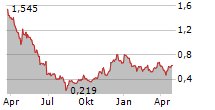
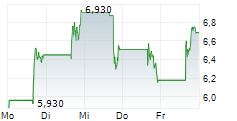
Das Unternehmen UNICYCIVE THERAPEUTICS INC kann der Branche Biotechnologie zugeordnet werden. Die relative Kursveränderung beträgt aktuell +0,53 % bei einem Aktienkurs von 0,640 Euro.
VerkaufenHaltenKaufen
■ Verkaufen
■ Halten
■ Kaufen
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und .
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu UNICYCIVE THERAPEUTICS INC finden Sie auf Wallstreet Online.